More Information
Submitted: December 12, 2024 | Approved: December 17, 2024 | Published: December 18, 2024
How to cite this article: Alimoradi H, Mashhadi F, Hemmat A, Nematy M, Khosravi M, Emadzadeh M, et al. Relationship between Fertility Diet Score Index Items and Ovulation in Women with Polycystic Ovary Syndrome: A Narrative Review. Arch Food Nutr Sci. 2024; 8(1): 041-048. Available from:
https://dx.doi.org/10.29328/journal.afns.1001061
DOI: 10.29328/journal.afns.1001061
Copyright License: © 2024 Alimoradi H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: PCOS; Infertility; Women; Fertility diet score; Fertility diet; Fertility; Diet
Relationship between Fertility Diet Score Index Items and Ovulation in Women with Polycystic Ovary Syndrome: A Narrative Review
Hadis Alimoradi1, Faezeh Mashhadi1, Ava Hemmat1, Mohsen Nematy1, Maryam Khosravi1, Maryam Emadzadeh2, Nayere Khadem Ghaebi3 and Fatemeh Roudi1*
1Department of Nutrition, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2Clinical Research Development Unit, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran
3Women's Health Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
*Address for Correspondence: Fatemeh Roudi, Department of Nutrition, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran, Email: [email protected]
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder affecting women of reproductive age, characterized by ovarian dysfunction and a leading cause of infertility due to ovulatory issues. Lifestyle interventions, including dietary modifications, exercise, and weight management, are considered first-line therapies for women with PCOS; however, the optimal treatment remains unidentified. The Fertility Diet (FD), introduced in 2007, represents a dietary approach that may positively impact fertility by emphasizing specific micronutrients, dietary composition modifications, weight management, and increased physical activity. This narrative review aims to evaluate how various components of the Fertility Diet influence ovulation and overall fertility, assessed through a fertility diet score. The findings of this study suggest that adherence to the Fertility Diet, particularly higher intake of the monounsaturated to trans-fat ratio, and increased vegetable protein intake, may positively influence fertility outcomes in individuals with PCOS. In contrast, high consumption of animal protein and high glycemic load food may have adverse effects. However, the current evidence remains insufficient for definitive conclusions, warranting further interventional studies to explore this relationship.
Polycystic Ovary Syndrome (PCOS) is a prevalent endocrine disorder that affects approximately 20% of women of reproductive age. Women with PCOS often experience a range of health issues, including obesity, metabolic syndrome, Insulin Resistance (IR), type 2 diabetes, and complications during pregnancy. These factors collectively impact their overall health and quality of life [1]. Ovarian dysfunction remains the primary characteristic of this syndrome, making it the leading cause of anovulation associated with infertility [2].
Diagnosing PCOS is challenging due to its diverse symptoms. According to the Rotterdam criteria, a diagnosis of PCOS requires the presence of two of the following three conditions: oligomenorrhea/anovulation, clinical or biochemical hyperandrogenism, and the presence of polycystic ovaries (≥12 follicles per ovary measuring 2–9 mm) [3].
Currently, the optimal treatment for PCOS remains uncertain. However, lifestyle interventions, including dietary changes, exercise, and behavioral modifications, are recommended as first-line therapies. These interventions have been shown to improve clinical symptoms, particularly by enhancing insulin sensitivity [4]. Furthermore, adopting a dietary pattern that increases the intake of specific micronutrients and supports weight management may help prevent infertility related to ovulatory disorders [5]. The Fertility Diet (FD), introduced in 2007 through the Nurses’ Health Study II, provides a framework for such dietary modifications. Evidence from this study suggests that a "fertility diet" pattern is associated with a reduced risk of infertility caused by ovulatory disorders [6].
The purpose of this review is to comprehensively evaluate the effects of various components of the fertility diet on the pathophysiology of PCOS and to elucidate the relationships between these components and their impact on the clinical manifestations of this complex endocrine disorder.
Fertility diet score index
Over the past two decades, research on nutritional intake and fertility has demonstrated that a healthier diet during the preconception period is associated with improved fertility outcomes [7]. Variations in preconception nutrition can significantly influence the metabolic functions of oocytes, their quality, and the development of the resulting embryo. While earlier studies often concentrated on individual nutrients or single food groups, recent research emphasizes the importance of overall dietary patterns. This holistic approach more accurately reflects real-life eating habits and accounts for the complex interactions among different nutrients in the diet [8].
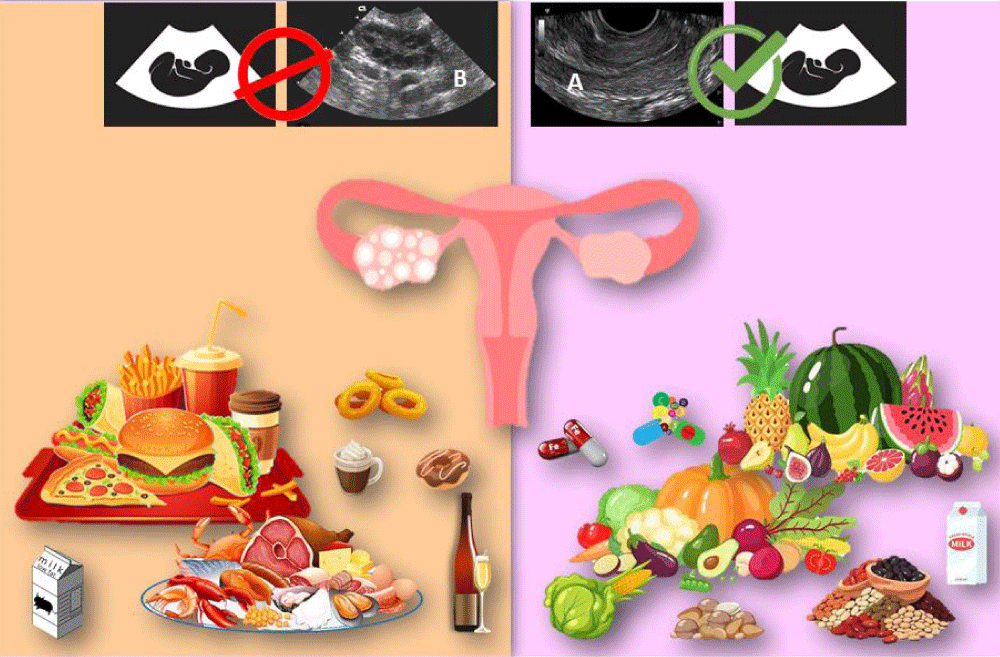
The fertility diet score is a comprehensive assessment that evaluates the collective impact of various nutrients, food items, and supplements on fertility. This dietary framework is based on research conducted by Chavarro, et al., which underscores the significant role that diet plays in reproductive outcomes, regardless of factors such as age, number of previous pregnancies, or BMI [9,10]. Their findings suggest that healthier eating patterns—characterized by a high intake of beans, whole grains, iron, vegetables, and fruits, along with reduced consumption of fast foods, sugary beverages, alcohol, and coffee—are linked to shorter times to conception, increased fertility rates, higher chances of clinical pregnancies and live births, and a lower risk of infertility due to ovulatory disorders, as illustrated in Figure 1 [10].
Figure 1: The effect of food items on fertility based on fertility diet score. A: Normal ovary view in ultrasound B: Polycystic ovary view in ultrasound.
This figure was created by the authors using free online resources and PowerPoint, and all images comply with their respective usage licenses.
Calculating the fertility diet score
According to Chavarro's study, the fertility diet score is calculated based on dietary items listed in Table 1, assigning points for each category according to their association with ovulatory disorder infertility. Each woman receives points ranging from 1 to 5, with higher scores reflecting healthier dietary choices. For factors such as the monounsaturated to trans fat ratio, vegetable protein intake, high-fat dairy consumption, iron, and multivitamins, points are assigned from 1 (lowest intake) to 5 (highest intake). Conversely, for animal protein, glycemic load, and low-fat dairy, the scoring is reversed; women in the lowest intake category receive 5 points while those in the highest category receive only 1 point. The total score is calculated by summing the points across all categories, resulting in a fertility diet score that ranges from 8 to 40. The median score observed in the study is 24 points. This structured scoring system provides a comprehensive evaluation of dietary patterns and their potential impact on reproductive health, offering practical insights for both research and clinical applications [10].
| Table 1: Criteria for determining Fertility diet score. | |||||
| Point assigned | |||||
| Dietary factor | 1 | 2 | 3 | 4 | 5 |
| Ratio of monounsaturated to trance fat | 6.3 less than | 6.3-7.3 | 7.4-8.2 | 8.3-9.6 | 9.6 more than |
| Animal protein (% of calories) | 17 more than | 14.9-17 | 13.4-16.9 | 11.6-13.3 | 11.5 less than |
| Vegetable protein (% of calories) | 4.1 less than | 4.1-4.6 | 4.7-5.1 | 5.2-5.7 | 5.7 more than |
| glycemic load | 139 more than | 128-139 | 118-127 | 107-117 | 107 less than |
| Multivitamin (tablets/wk) | 0 | 2 or less | unknown | 03-May | 6 or more |
| Iron (mg/day) | 12 less then | 12.1-15.6 | 15.7-27.1 | 27.2-54.3 | 54.3 more than |
| high-fat dairy (serving) | 1/wk or less | 4-2/wk | - | 5.6/wk | 1 or more |
| low-fat dairy (serving) | 2/wk or less | 1/d | 6-5/wk | 4-2/wk | 1/wk or less |
Dietary fatty acid
Dietary Fatty Acid (FA) patterns significantly influence complications associated with PCOS [11]. Fats are classified into three categories—Saturated Fatty Acids (SFA), Monounsaturated Fatty Acids (MUFA), and Polyunsaturated Fatty Acids (PUFA)—based on the length of their carbon chains and their degree of saturation [12]. SFAs are long-chain carboxylic acids without double bonds, typically consisting of 12-24 carbon atoms. In contrast, MUFAs have one unsaturated bond, while PUFAs have two or more unsaturated bonds in their straight-chain structure. Research suggests that a diet high in SFAs, commonly found in processed foods, fast foods, high-fat dairy products, and red meat, can elevate the risk of cardiovascular disease and metabolic syndrome. On the other hand, both MUFAs and PUFAs are known to have protective health benefits [13-15].
The predominant fatty acids found in the follicular fluid of both animals and humans include palmitic, oleic, and stearic acids, with linoleic acid being more prevalent in humans. Research using animal models indicates that SFAs, particularly palmitic and stearic acids, are linked to decreased fertilization rates, impaired cleavage, and reduced blastocyst development. Furthermore, these fatty acids inhibit the growth of granulosa and theca cells, promote apoptosis, and negatively affect ovarian function [16]. Evidence suggests that the consumption of SFAs is associated with inflammation and Insulin Resistance (IR) in individuals with PCOS. Intake of SFAs has been shown to disrupt insulin signaling and affect cell membrane function, leading to reduced insulin sensitivity. In contrast, MUFAs and PUFAs do not exhibit these detrimental effects [17].
Research indicates that diets rich in MUFAs can improve serum High-density Lipoprotein Cholesterol (HDL-C) and triglyceride levels, as well as enhance insulin sensitivity in women with PCOS. This is achieved through increased production of Sex Hormone-Binding Globulin (SHBG) and a reduction in free testosterone and insulin-stimulated androgen synthesis [18]. Unsaturated fatty acids, such as oleic acid, have been shown to enhance blastocyst formation, oocyte maturation, and embryo development. Oleic acid can also mitigate the negative effects of palmitic and stearic acids, improving post-fertilization developmental competence without any adverse impacts. In contrast, PUFAs, such as linoleic acid, may have a more nuanced effect on fertility, with some studies suggesting a positive correlation with fertility rates, while others indicate it may negatively affect oocyte maturation and developmental competence [16].
Some studies suggest that substituting SFAs with PUFAs can help reduce IR and offer benefits for chronic disorders and inflammation, both of which play a crucial role in the development of PCOS. However, diets rich in PUFAs may not be as effective as those high in MUFAs. Research involving women with PCOS has shown that increasing PUFA intake can result in elevated fasting glucose levels without significant changes in insulin, blood lipids, testosterone, or Sex Hormone-Binding Globulin (SHBG) levels [17-19]. Nevertheless, considering that a substantial proportion of women with PCOS consume high amounts of SFA, any reduction in total fat intake should prioritize decreasing SFA while maintaining adequate consumption of MUFA and PUFA [17].
Carbohydrate
Both lean and overweight women with PCOS often struggle with weight management due to the anabolic effects of insulin and alterations in energy expenditure and food intake, compounded by elevated androgen levels that increase cravings for high-GI (glycemic index) foods [20,21]. The Glycemic Index (GI) measures the rate at which carbohydrates raise blood sugar levels. Low-GI foods (e.g., oatmeal, carrots, non-starchy vegetables, and apples) elevate blood sugar levels gradually, while high-GI foods (e.g., white rice, honey, and sugar-rich foods) cause a rapid spike [22,23]. Acute hyperglycemia induced by oral glucose loading increases inflammation and oxidative stress, subsequently leading to insulin resistance through various mechanisms [24].
Dietary modification plays a crucial role in managing PCOS. To date, the optimal dietary strategy to mitigate weight gain in this population remains unclear [20]. It has been suggested that a Low Glycemic Index (LGI) diet can improve the anthropometric and metabolic characteristics of overweight women with PCOS, regulate menstrual cycles, and reduce hyperandrogenism [25]. LGI diets decrease appetite, enhance fat oxidation, reduce fat accumulation, and lower insulin secretion, thus improving insulin resistance and decreasing androgen levels [26,27]. However, evidence indicates that a high glycemic index diet can stimulate insulin synthesis, even if patients do not consume excessive daily calories. This can lead to an increased hepatic synthesis of Insulin-like Growth Factor 1 (IGF-I) [25]. Consequently, novel strategies aimed at reducing hyperinsulinemia may have significant implications for promoting weight loss, improving insulin resistance, and decreasing androgen levels in women with PCOS and infertility [28].
A key strategy for promoting health through an LGI diet involves incorporating non-starchy vegetables, legumes, and LGI fruits such as apples, pears, peaches, and berries, which provide significant benefits. Tropical fruits like bananas, mangoes, and papayas can also be healthier alternatives to high-GI desserts. Whole grains in minimally processed forms, such as whole-kernel bread, brown rice, stone-ground bread, and steel-cut oats, are recommended, while refined grains like white bread, pasta, and white potatoes should be limited. High-calorie foods with a low GI, such as ice cream, should be consumed occasionally, while sugar-sweetened beverages should be avoided entirely, and fruit juice intake minimized. Meals should include healthy protein sources such as legumes, fish, or skinless poultry, complemented by healthy fats from olive oil, nuts, and avocados, consumed in moderation. Saturated fats from animal and dairy products should be limited, and trans fats completely avoided. A regular eating pattern, including three balanced meals and one or two healthy snacks per day, is recommended. Emphasis should also be placed on mindful eating practices, such as eating slowly and stopping when feeling full [25,29-31].
Protein
Normal Protein (NP) diets (15% protein, 55% carbohydrate, 30% lipid) and High-Protein (HP) diets (30% protein, 40% carbohydrate, 30% fat) have been employed to manage weight and improve metabolic and reproductive aspects in women with PCOS. HP diets have been shown to facilitate greater weight loss and maintenance compared to NP diets. They enhance insulin sensitivity, lower levels of high-sensitivity C-Reactive Protein (hs-CRP), boost basal metabolism and postprandial energy expenditure and decrease postprandial glucose levels [32,33].
However, evidence suggests that the consumption of animal protein may increase the risk of ovulatory infertility while replacing animal proteins—particularly chicken and red meat—with plant-based proteins could reduce the risk of infertility due to anovulation [32]. Another potential explanation for these observed connections may lie in the differing impacts of animal and plant-based proteins on the levels of IGF-I in the bloodstream. Elevated free IGF-I levels may contribute to the pathophysiology of PCOS. A higher intake of animal protein leads to a more acidic body environment, which is linked to increased insulin resistance. Consequently, consuming high-protein sources, particularly those derived from plants, can effectively improve conditions related to PCOS and infertility [34].
Dairy products
Dairy product consumption, particularly the type of dairy, may significantly impact fertility and ovulation [35]. Dairy products have a complex relationship with carbohydrate metabolism, Insulin Resistance (IR), type 2 diabetes, and ovulatory function. The effects of dairy vary based on fat content and specific components, including amino acids, fatty acids, and minerals [36]. When comparing different types of dairy, high-fat dairy products have been shown to contain more estrogen and contribute to a smaller increase in serum IGF-1 concentrations compared to low-fat products [37,38]. Another study found that consuming skim milk was associated with a higher prevalence of acne—a common condition among those with PCOS—possibly due to the presence of androgen precursors in milk [39]. Therefore, for women with PCOS at risk, dietary modifications, such as opting for high-fat dairy products instead of low-fat options, may influence ovulatory infertility [36,39].
However, some studies report conflicting results regarding the relationship between dairy products and carbohydrate metabolism in individuals with PCOS. Several investigations suggest that higher consumption of milk and dairy products is linked to elevated HOMA-IR values, indicating a potential adverse effect on insulin resistance [28,34,38]. A meta-analysis reported a significant increase in fasting blood glucose levels associated with higher dairy intake, although fasting insulin levels and HOMA-IR did not show significant changes [40]. In contrast, two other studies highlighted the beneficial effects of dairy consumption, particularly low-fat dairy products on HOMA-IR, waist circumference, and body weight [41,42].
Consequently, given these conflicting results, it remains unclear whether dairy products in managing PCOS. It is also essential to note that research specifically examining the effects of milk consumption in women with PCOS is limited. Therefore, the purported benefits of milk consumption in this patient group cannot be definitively confirmed. Conducting comprehensive studies on the effects of dairy consumption in women with PCOS is crucial for understanding its potential benefits [36].
Iron
Iron levels have a significant impact on fertility in women with PCOS, with both deficiency and excess presenting challenges to reproductive health. Insufficient iron can result in anemia, which may disrupt ovulation and reduce overall fertility. On the other hand, an overload of iron can lead to oxidative stress and inflammation, potentially exacerbating the symptoms of PCOS and adversely affecting the quality of oocytes [43,44]. Most women with PCOS exhibit mild iron overload, which can arise from various factors. One contributing factor may be linked to irregular menstrual cycles and elevated androgen levels. The impact of hyperandrogenemia on estimated body iron stores could stem from two primary mechanisms: the stimulatory effect of androgens on erythropoiesis, which enhances intestinal iron absorption, and the reduced menstrual blood loss resulting from chronic menstrual dysfunction commonly observed in PCOS. Additionally, iron overload in individuals with PCOS may be associated with oxidative stress, which stimulates increased ferritin synthesis. Elevated serum ferritin levels in these patients correlate with Insulin Resistance (IR). Notably, IR in PCOS may enhance iron absorption and decrease hepcidin levels. Consequently, iron overload negatively influences fertility in women with PCOS through hormonal imbalances, worsened insulin resistance, oxidative stress, compromised endometrial health, and increased inflammation to maintain iron homeostasis in individuals at risk of overload, it is essential to monitor dietary and genetic factors. Therefore, tracking these influences is crucial for maintaining optimal iron balance [45-47].
Multivitamins
Adequate intake of vitamins and minerals, such as B vitamins (including B6, B12, and B9), along with antioxidant vitamins A, C, and E, as well as vitamin D, has been shown to positively impact female ovulatory fertility and PCOS [48].
B vitamins: Chavarro, et al. found that women who consumed multivitamin supplements at least three times a week experienced a lower risk of anovulatory infertility. Folic acid levels appear to be significantly linked to Insulin Resistance (IR) and PCOS. Research indicates that a daily intake of 700 μg of folic acid can decrease the risk of ovulatory disorders by 40% - 50% [10,49]. Folic acid positively influences female fertility primarily by reducing oxidative stress and regulating the production of pro-inflammatory cytokines. These factors significantly affect ovulation and the development of oocytes. Additionally, folic acid may impact ovulation by reducing the ovarian response to FSH stimulation when serum folate levels are low. It works alongside vitamins B6 and B12 to manage homocysteine levels. In women with PCOS, studies have shown that folic acid supplementation can lower homocysteine levels, potentially improve metabolic profiles, and alleviate some characteristic symptoms of PCOS [49,50].
Antioxidants
Vitamin E: In women with PCOS, the positive effects of vitamin E on glycemic control are attributed to its antioxidant properties. Oxidative stress can lead to increased hemoglobin glycation and elevated blood glucose levels. Furthermore, vitamin E supplementation has been shown to lower testosterone and Luteinizing Hormone (LH) levels while increasing progesterone and Follicle-Stimulating Hormone (FSH) levels [51]. However, conflicting evidence exists regarding its efficacy in enhancing dominant follicle counts and overall pregnancy rates, suggesting that while it may support certain reproductive functions and reduce treatment-related costs, its overall impact remains limited [52-54].
Vitamin C: Vitamin C is essential for regulating the menstrual cycle and supporting ovarian function. The excretion of ascorbic acid increases before ovulation, decreases just before ovulation, and then rises again immediately following the post-ovulation temperature spike. This fluctuation reflects the absorption of ascorbic acid in the pre-ovulatory ovary, which is crucial for facilitating proper ovulation. Elevated levels of ascorbic acid stimulate the production of hormones such as progesterone and oxytocin and are found in high concentrations in the corpus luteum. Additionally, significant amounts of ascorbic acid in the ovaries contribute to collagen synthesis, which is vital for the growth of follicles and the corpus luteum, as well as for recovery and repair after ovulation [54,55].
Moreover, studies highlight the positive impact of vitamin C supplementation during fertility treatments, leading to elevated progesterone levels and improved follicular fluid concentrations. These changes may enhance endometrial receptivity and improve outcomes for women facing infertility challenges [56,57]. Overall, maintaining adequate vitamin C intake is vital for reproductive health and could be a valuable component in infertility management.
Vitamin D: Serum Anti-Müllerian Hormone (AMH) levels are elevated in women with PCOS, making AMH a useful diagnostic marker for this condition. Vitamin D therapy has been shown to lower these elevated AMH levels, indicating a potential role for vitamin D in regulating ovarian function. Furthermore, vitamin D supplementation can increase the levels of anti-inflammatory soluble receptors for advanced glycation end-products, which may enhance insulin sensitivity in vitamin D-deficient women with PCOS, thereby further contributing to the regulation of ovulation. Additionally, vitamin D plays a significant role in enhancing ovulation and improving fertility by modulating reproductive functions through its receptors in reproductive tissues, including the ovaries [50]. This multifaceted approach suggests that incorporating vitamin D supplementation into treatment plans could be beneficial for effectively managing PCOS symptoms [50,58,59].
Limitation and suggestion
Investigating the impact of individual components of a fertility diet on the pathophysiology of Polycystic Ovary Syndrome (PCOS) offers opportunities to improve fertility outcomes in affected individuals. Nevertheless, the available evidence remains insufficient to draw definitive conclusions. Although some studies indicate that following a fertility diet can enhance ovulation and increase pregnancy rates, there is still a lack of robust research that specifically connects dietary patterns to reproductive health outcomes in women with PCOS.
This limitation emphasizes the importance of conducting larger and more rigorous intervention studies, along with long-term research, to clarify the mechanisms behind these relationships and establish causal connections between diet and fertility results. Future studies should also focus on evaluating the individual elements of fertility diets in detail, exploring their specific effects and interactions across different PCOS phenotypes.
Addressing these research gaps will provide critical insights for clinical practitioners, support evidence-based dietary recommendations, and ultimately improve fertility care and outcomes for women with PCOS.
The findings of this study indicate that adherence to fertility diet items can positively influence fertility outcomes in women with PCOS. Specifically, a higher intake of monounsaturated fats, certain vitamins, and vegetable proteins is associated with improved ovulatory function and fertility, whereas excessive consumption of animal proteins and foods with a high glycemic load may have adverse effects. These insights highlight the potential of dietary modifications in enhancing reproductive health.
However, the current evidence is insufficient to confirm the definitive impact of the Fertility Diet on fertility outcomes in PCOS. Future large-scale, well-designed intervention studies are required to establish clear causal relationships and provide actionable dietary guidelines for clinical practice. Addressing these gaps will contribute significantly to improving patient care and fertility management in PCOS.
- Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071-e83. Available from: https://doi.org/10.1210/clinem/dgaa839
- Mohammad MB, Seghinsara AM. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pac J Cancer Prev. 2017;18(1):17-21. Available from: https://doi.org/10.22034/apjcp.2017.18.1.17
- Greff D, Juhász AE, Váncsa S, Váradi A, Sipos Z, Szinte J, et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. 2023;21(1):10. Available from: https://doi.org/10.1186/s12958-023-01055-z
- Saadati N, Haidari F, Barati M, Nikbakht R, Mirmomeni G, Rahim F. The effect of low glycemic index diet on the reproductive and clinical profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Heliyon. 2021;7(11):e08338. Available from: https://doi.org/10.1016/j.heliyon.2021.e08338
- Saeed SA, Kareem HH. Diet and lifestyle in the prevention of ovulatory disorder infertility. World J Pharm Res. 2019;8(4). Available from: https://www.wisdomlib.org/science/journal/world-journal-of-pharmaceutical-research/d/doc1376288.html
- Eskew AM, Bedrick BS, Chavarro JE, Riley JK, Jungheim ES. Dietary patterns are associated with improved ovarian reserve in overweight and obese women: a cross-sectional study of the Lifestyle and Ovarian Reserve (LORe) cohort. Reprod Biol Endocrinol. 2022;20(1):33. Available from: https://doi.org/10.1186/s12958-022-00907-4
- Habibi N, Hall KA, Moran LJ, Haag DG, Hodge AM, Grieger JA. Is the association between age and fertility problems modified by diet quality? Findings from a national study of reproductive age women in Australia. Nutrients. 2022;14(20):4355. Available from: https://doi.org/10.3390/nu14204355
- Jahangirifar M, Taebi M, Nasr-Esfahani MH, Askari G. Dietary patterns and the outcomes of assisted reproductive techniques in women with primary infertility: a prospective cohort study. Int J Fertil Steril. 2019;12(4):316-323. Available from: https://doi.org/10.22074/ijfs.2019.5373
- Ghasemisedaghat S, Eslamian G, Kazemi SN, Rashidkhani B, Taheripanah R. Association of fertility diet score with endometriosis: a case-control study. Front Nutr. 2023;10:1222018. Available from: https://doi.org/10.3389/fnut.2023.1222018
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110(5):1050-8. Available from: https://doi.org/10.1097/01.aog.0000287293.25465.e1
- Yahay M, Heidari Z, Allameh Z, Amani R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: a randomized controlled trial. Lipids Health Dis. 2021;20:7. Available from: https://doi.org/10.1186/s12944-021-01433-9
- Polak A, Krentowska A, Łebkowska A, Buczyńska A, Adamski M, Adamska-Patruno E, et al. The association of serum levels of leptin and ghrelin with the dietary fat content in non-obese women with polycystic ovary syndrome. Nutrients. 2020;12(9):E2753. Available from: https://doi.org/10.3390/nu12092753
- Selvan T, Nagarajan G. Combustion and emission characteristics of a diesel engine fuelled with biodiesel having varying saturated fatty acid composition. Int J Green Energy. 2013;10(9):952-65. Available from: http://dx.doi.org/10.1080/15435075.2012.732157
- Rani MP, Bahuleyan K. Chemistry and therapeutic potential of plant-based fatty acids. In: Traditional Medicines in Drug Discovery and Development. 2024:362. Available from: https://www.cambridgescholars.com/resources/pdfs/978-1-0364-0345-4-sample.pdf
- Ruiz-Núñez B, Dijck-Brouwer DJ, Muskiet FA. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem. 2016;36:1-20. Available from: https://doi.org/10.1016/j.jnutbio.2015.12.007
- Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, et al. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis. 2017;16:18. Available from: https://doi.org/10.1186/s12944-016-0396-z
- Jeanes YM, Reeves S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: diagnostic and methodological challenges. Nutr Res Rev. 2017;30(1):97-105. Available from: https://doi.org/10.1017/s0954422416000287
- Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85(3):679-88. Available from: https://doi.org/10.1016/j.fertnstert.2005.08.045
- Unfer V. A deeper assessment of ω3-poly-unsaturated fatty acids in polycystic ovary syndrome management. Comment on Regidor et al. Chronic inflammation in PCOS: The potential benefits of specialized pro-resolving lipid mediators (SPMs) in the improvement of the resolutive response. Int J Mol Sci. 2021;22(18):10114. Available from: https://doi.org/10.3390/ijms221810114
- Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism. 2014;63(10):1257-64. Available from: https://doi.org/10.1016/j.metabol.2014.07.007
- Larsson I, Hulthén L, Landén M, Pålsson E, Janson P, Stener-Victorin E. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr. 2016;35(1):213-8. Available from: https://doi.org/10.1016/j.clnu.2015.02.006
- Care D. Standards of care in diabetes—2023. Diabetes Care. 2023;46:S1-S267.
- MacLeod J, Franz MJ, Handu D, Gradwell E, Brown C, Evert A, et al. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Nutrition Intervention Evidence Reviews and Recommendations. J Acad Nutr Diet. 2017;117(10):1637-58. Available from: https://doi.org/10.1016/j.jand.2017.03.023
- Zhang J, Liu Y, Liu X, Xu L, Zhou L, Tang L, et al. High intake of energy and fat in Southwest Chinese women with PCOS: a population-based case-control study. PLoS One. 2015;10(5):e0127094. Available from: https://doi.org/10.1371/journal.pone.0127094
- Shishehgar F, Mirmiran P, Rahmati M, Tohidi M, Ramezani Tehrani F. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocr Disord. 2019;19(1):1-11. Available from: https://doi.org/10.1186/s12902-019-0420-1
- Altieri P, Cavazza C, Pasqui F, Morselli AM, Gambineri A, Pasquali R. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2013;78(1):52-9. Available from: https://doi.org/10.1111/j.1365-2265.2012.04355.x
- Farshchi H, Rane A, Love A, Kennedy R. Diet and nutrition in polycystic ovary syndrome (PCOS): pointers for nutritional management. J Obstet Gynaecol. 2007;27(8):762-73. Available from: https://doi.org/10.1080/01443610701667338
- Phy JL, Pohlmeier AM, Cooper JA, Watkins P, Spallholz J, Harris KS, et al. Low starch/low dairy diet results in successful treatment of obesity and co-morbidities linked to polycystic ovary syndrome (PCOS). J Obes Weight Loss Ther. 2015;5(2):259. Available from: https://doi.org/10.4172/2165-7904.1000259
- Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92(1):83-92. Available from: https://doi.org/10.3945/ajcn.2010.29261
- Manta A, Paschou SA, Isari G, Mavroeidi I, Kalantaridou S, Peppa M. Glycemic index and glycemic load estimates in the dietary approach of polycystic ovary syndrome. Nutrients. 2023;15(15):3483. Available from: https://doi.org/10.3390/nu15153483
- Kazemi M, Hadi A, Pierson RA, Lujan ME, Zello GA, Chilibeck PD. Effects of dietary glycemic index and glycemic load on cardiometabolic and reproductive profiles in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2021;12(1):161-78. Available from: https://doi.org/10.1093/advances/nmaa092
- Toscani MK, Mario FM, Radavelli-Bagatini S, Wiltgen D, Cristina Matos M, Spritzer PM. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: a randomized study. Gynecol Endocrinol. 2011;27(11):925-30. Available from: https://doi.org/10.3109/09513590.2011.564686
- Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31(2):117-25. Available from: https://doi.org/10.1080/07315724.2012.10720017
- Lawlor D, Ebrahim S, Timpson N, Davey Smith G. Avoiding milk is associated with a reduced risk of insulin resistance and the metabolic syndrome: findings from the British Women's Heart and Health Study. Diabet Med. 2005;22(6):808-11. Available from: https://doi.org/10.1111/j.1464-5491.2005.01537.x
- Chavarro J, Rich-Edwards J, Rosner B, Willett W. A prospective study of dairy foods intake and anovulatory infertility. Hum Reprod. 2007;22(5):1340-7. Available from: https://doi.org/10.1093/humrep/dem019
- Janiszewska J, Ostrowska J, Szostak-Węgierek D. Milk and dairy products and their impact on carbohydrate metabolism and fertility—a potential role in the diet of women with polycystic ovary syndrome. Nutrients. 2020;12(11):3491. Available from: https://doi.org/10.3390/nu12113491
- Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379-89. Available from: https://doi.org/10.1016/j.ajog.2017.08.010
- Tucker LA, Erickson A, LeCheminant JD, Bailey BW. Dairy consumption and insulin resistance: the role of body fat, physical activity, and energy intake. J Diabetes Res. 2015;2015:206959. Available from: https://doi.org/10.1155/2015/206959
- Rajaeieh G, Marasi M, Shahshahan Z, Hassanbeigi F, Safavi SM. The relationship between intake of dairy products and polycystic ovary syndrome in women who were referred to Isfahan University of Medical Science Clinics in 2013. Int J Prev Med. 2014;5(6):687-94. Available from: https://pubmed.ncbi.nlm.nih.gov/25013687/
- O'Connor S, Turcotte A-F, Gagnon C, Rudkowska I. Increased dairy product intake modifies plasma glucose concentrations and glycated hemoglobin: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10(2):262-79. Available from: https://doi.org/10.1093/advances/nmy074
- Sochol KM, Johns TS, Buttar RS, Randhawa L, Sanchez E, Gal M, et al. The effects of dairy intake on insulin resistance: a systematic review and meta-analysis of randomized clinical trials. Nutrients. 2019;11(9):2237. Available from: https://doi.org/10.3390/nu11092237
- Rastad H, Shahrestanaki E, Heydarian HR, Maarefvand M. Dairy consumption and its association with anthropometric measurements, blood glucose status, insulin levels, and testosterone levels in women with polycystic ovary syndrome: a comprehensive systematic review and meta-analysis. Front Endocrinol (Lausanne). 2024;15:1334496. Available from: https://doi.org/10.3389/fendo.2024.1334496
- Orisaka M, Mizutani T, Miyazaki Y, Shirafuji A, Tamamura C, Fujita M, et al. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front Endocrinol (Lausanne). 2023;14:1324429. Available from: https://doi.org/10.3389/fendo.2023.1324429
- Skoracka K, Ratajczak AE, Rychter AM, Dobrowolska A, Krela-Kaźmierczak I. Female fertility and the nutritional approach: the most essential aspects. Adv Nutr. 2021;12(6):2372-86. Available from: https://doi.org/10.1093/advances/nmab068
- Behboudi-Gandevani S, Abtahi H, Saadat N, Tohidi M, Ramezani Tehrani F. Effect of phlebotomy versus oral contraceptives containing cyproterone acetate on the clinical and biochemical parameters in women with polycystic ovary syndrome: a randomized controlled trial. J Ovarian Res. 2019;12:78. Available from: https://doi.org/10.1186/s13048-019-0554-9
- Mathew M, Sivaprakasam S, Phy JL, Bhutia YD, Ganapathy V. Polycystic ovary syndrome and iron overload: biochemical link and underlying mechanisms with potential novel therapeutic avenues. Biosci Rep. 2023;43(1):BSR20212234. Available from: https://doi.org/10.1042/bsr20212234
- Ko PC, Huang SY, Hsieh CH, Hsu MI, Hsu CS. Serum ferritin levels and polycystic ovary syndrome in obese and nonobese women. Taiwanese J Obstet Gynecol. 2015;54(4):403-7. Available from: https://doi.org/10.1016/j.tjog.2014.06.005
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertil Steril. 2008;89(3):668-76. Available from: https://doi.org/10.1016/j.fertnstert.2007.03.089
- Gaskins AJ, Mumford SL, Chavarro JE, Zhang C, Pollack AZ, Wactawski-Wende J, et al. The impact of dietary folate intake on reproductive function in premenopausal women: a prospective cohort study. PLoS One. 2012;7(9):e46276. Available from: https://doi.org/10.1371/journal.pone.0046276
- Jurczewska J, Szostak-Węgierek D. The influence of diet on ovulation disorders in women—a narrative review. Nutrients. 2022;14(8):1556. Available from: https://doi.org/10.3390/nu14081556
- Tefagh G, Payab M, Qorbani M, Sharifi F, Sharifi Y, Ebrahimnegad Shirvani MS, et al. Effect of vitamin E supplementation on cardiometabolic risk factors, inflammatory and oxidative markers and hormonal functions in PCOS (polycystic ovary syndrome): a systematic review and meta‐analysis. Sci Rep. 2022;12(1):5770. Available from: https://doi.org/10.1038/s41598-022-09082-3
- Gül DK, Şolt A. The effect of vitamin E supplements added to Clomiphene citrate treatment on fertility in polycystic ovary syndrome. Celal Bayar Univ Health Sci Inst J. 2021;8(3):443-8. Available from: https://doi.org/10.34087/cbusbed.843323
- Chen J, Guo Q, Pei Y-h, Ren Q-l, Chi L, Hu R-k, et al. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: a retrospective cohort study. BMC Women's Health. 2020;20:1-9. Available from: https://doi.org/10.1186/s12905-020-00930-w
- Iervolino M, Lepore E, Forte G, Laganà A, Buzzaccarini G, Unfer V. Natural Molecules in the Management of Polycystic Ovary Syndrome (PCOS): An Analytical Review. Nutrients. 2021;13(5):1677. Available from: https://doi.org/10.3390/nu13051677
- Olaniyan OT, Femi A, Iliya G, Ayobami D, Godam E, Olugbenga E, et al. Vitamin C suppresses ovarian pathophysiology in experimental polycystic ovarian syndrome. Pathophysiology. 2019;26(3-4):331-41. Available from: https://doi.org/10.1016/j.pathophys.2019.08.003
- Henmi H, Endo T, Kitajima Y, Manase K, Hata H, Kudo R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertil Steril. 2003;80(2):459-61. Available from: https://doi.org/10.1016/s0015-0282(03)00657-5
- Brilliant A, Astuti BPK, Joyo EO, Febri RR, Silvana V, Muharam R. Vitamin B3 (niacin), B6, C, and iron intake are associated with the free androgen index, especially in normoandrogenic polycystic ovary syndrome. J Turk Ger Gynecol Assoc. 2022;23(3):130-6. Available from: https://doi.org/10.4274/jtgga.galenos.2022.2022-2-1
- Mohan A, Haider R, Fakhor H, Hina F, Kumar V, Jawed A, et al. Vitamin D and polycystic ovary syndrome (PCOS): A review. Ann Med Surg. 2023;85(7):3506-11. Available from: https://doi.org/10.1097/ms9.0000000000000879
- Lerchbaum E, Theiler-Schwetz V, Kollmann M, Wölfler M, Pilz S, Obermayer-Pietsch B, et al. Effects of vitamin D supplementation on surrogate markers of fertility in PCOS women: a randomized controlled trial. Nutrients. 2021;13(2):547. Available from: https://doi.org/10.3390/nu13020547