More Information
Submitted: July 08, 2023 | Approved: July 19, 2023 | Published: July 20, 2023
How to cite this article: Iji M, Yamada K, Yamane Y, Watanabe C, Takemoto K, et al. Improvement of the Deterioration of the Gut Microbiota Due to High-fat, High-sucrose Diet Acylated Steryl-β-glycosides Intake. Arch Food Nutr Sci. 2023; 7: 065-069.
DOI: 10.29328/journal.afns.1001051
Copyright License: © 2023 Iji M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: High-fat diet; Plant sterol; Intestinal bacterial flora; Obese; Body fat
Abbreviations: ASG: Acylated Steryl-β-Glycosides; HFD: High-Fat Diets
Improvement of the Deterioration of the Gut Microbiota Due to High-fat, High-sucrose Diet Acylated Steryl-β-glycosides Intake
Masaki Iji1, Kuniyuki Yamada1, Yuta Yamane1, Chihiro Watanabe1, Kazuhito Takemoto1, Mamoru Tanaka2, Yuichiro Takei1, Takako Miyaue1, Yoichi Miura1 and Hiroyuki Watanabe1*
1Graduate School of Human Life Sciences, University of Kochi, Japan
2Department of Food and Nutritional Sciences, Chubu University, Japan
*Address for Correspondence: Hiroyuki Watanabe, Graduate School of Human Life Sciences, University of Kochi, Kochi, Japan, Email: [email protected]
Daily high-fat diet (HFD) intake is generally associated with an increased risk of metabolic diseases, cancer, and neurological disorders, which represent a major global health burden with significant social and economic consequences. In the present study, mice were treated with HFD containing 40% lipids. Furthermore, HFD was supplemented with 0.5% or 1.0% acylated sterol-β-glucoside (ASG).
After 55 days of rearing, body weight, epididymal fat weight, weight, and pH of cecum contents and intestinal microflora were compared with mice fed HFD or a low-fat diet (LFD) containing lipid at 7%. The results showed that body weight and epididymis fat weight on the last day of feeding were significantly higher in HFD, 0.5% ASG, and 1.0% ASG compared to LFD, but significantly lower in 0.5% ASG and 1.0% ASG compared to HFD. Cecum content weight was lower with HFD compared to LFD but increased to LFD levels with the addition of ASG. Cecum pH was significantly lower on the 1.0% ASG compared to the other groups.
The gut microbiota was significantly elevated in the HFD compared to the LFD, with Bacilliota specific to obese mice. However, the addition of ASG to the HFD decreased the Bacilliota and increased the Bacteroidota. Clostridium cluster XI and Clostridium subcluster XIVa, intestinal bacteria involved in the production of carcinogenic secondary bile acids, were markedly increased by consumption of the HFD but were markedly decreased by ASG.
Daily intake of ASG may inhibit the deterioration of gut bacteria caused by HFD and reduce the disease risk posed by HFD.
Diets high in fat, particularly saturated fat, are commonly consumed but have serious public health implications. High-fat diets (HFDs) are associated with metabolic diseases, cancer, and neurological disorders [1-3]. Furthermore, these findings indicate a relationship between dietary lipids and their impact on the gut microbiota. Thus, the intake of dietary fat and saturated fatty acids influences the composition of the gut microbiota, with a lower proportion of Bacteroidetes and a higher proportion of Firmicutes in the intestinal tract of mice fed with a high-fat, high-sugar diet compared with mice fed with a low-fat, high-sugar diet. Particularly, this can cause the reduction of useful bacteria and the possibility of an increase in disease-inducing bacteria [4].
Typical plant sterols contain campesterol, β-sitosterol, and stigmasterol, which differ at the C-3 position of their steroid skeleton and are found either as free sterols with a free hydroxyl group, or sterol esters that are esters of fatty acids, steryl glycosides, or acylated steryl-β-glycosides (ASG) that are acyl groups attached to a sugar [5].
Dietary plant sterols, such as cholesterol form micelles with bile acids and are absorbed into the body. The absorption rate of plant sterols varies according to the type of sterol, but it is a maximum of 10% and rarely absorbed and excreted in the feces [6]. Furthermore, during the absorption of cholesterol in the gastrointestinal tract, plant sterols compete with cholesterol to dissolve into bile acid micelles, thereby lowering the amount of cholesterol absorbed by small intestinal epithelial cells, which is known to decrease the concentration of cholesterol in the blood [7].
Furthermore, the effects of plant sterols migrating into the gastrointestinal tract on the gut microbiota cannot be ignored. The characteristics of the dynamics of plant sterols in the intestinal tract may be one of the mechanisms by which plant sterol intake and changes in the gut microbiota are associated with improvements in obesity and diabetes mellitus [8]. Therefore, the effects of plant sterols migrating into the gastrointestinal tract on gut microbiota cannot be ignored.
Therefore, the aim of the present study was to investigate the effects of ASG, a phytosterol derivative, on the gut microbiota of obese mice induced by a high-fat, high-sucrose diet.
ASG was extracted from rice bran and purified to 91% purity using a silica gel column. The sterol composition (W/W) was 79.4% β-sitosterol, 12.3% campesterol, 8.2% stigmasterol, and 0.1% other. The acyl group composition (W/W) was 77.54% palmitic acid, 15.54% oleic acid, and 7.31% linoleic acid.
The experimental diets were based on AIN 93G, a high-fat, high-sucrose diet (HFD) containing 30% lard and 10% vegetable oil with 13% sucrose and either 0.5% or 1.0% ASG. Mice fed with a low-fat diet (LFD) containing 7% fat and no sucrose belonged to the control group. There were eight animals in each group.
The study was approved by the Animal Experimentation Committee of the University of Kochi (approval number 2018-03). The animal laboratory was kept at a room temperature of 24 °C ± 2 °C, humidity of 55% ± 3%, and a 12-h light/dark cycle. The experimental animals used in the study were 8-week-old C57BL/6J male mice (Japan SLC, Shizuoka, Japan). Food intake was recorded daily, and pair-feeding methods were used to ensure that each group of mice ingested the same number of calories. On the 55th day of feeding, the animals were killed by cardiac bloodletting under isoflurane anesthesia inhalation without fasting. Epididymal fat and cecum were collected and weighed, and the cecum contents were stored at −30 °C until cecal flora gene analysis. Analysis of the cecal microflora by the T-RFLP method was contracted to Techno Suruga Laboratory Ltd and was performed according to the protocol described in the method of Nagashima, et al. [9].
Statistical analysis
The obtained values for each group are expressed as mean ± standard deviation. We applied the Bell Curve software for Excel ver. 3.21 (Social Survey). After conducting an analysis of variance, we compared the groups using Tukey’s multiple comparison tests and determined their correlations by analyzing each study item via regression analysis using the values of the significance level was set at 5% or 1% in the two-sided test.
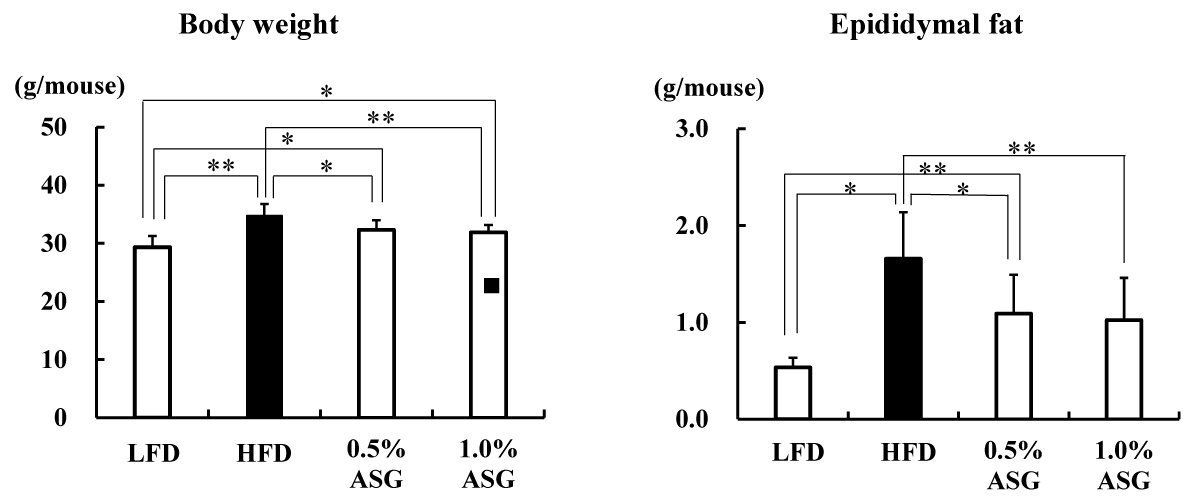
The body weight was significantly greater in HFD (p < 0.01), 0.5% ASG (p < 0.05), and 1.0% ASG (p < 0.05) than in LFD on the last day of rearing. However, significantly lower values were observed in 0.5% ASG (p < 0.05), and 1.0% ASG (p < 0.01) than in HFD, with no significant differences between 0.5% ASG and 1.0% ASG (Figure 1).
Figure 1: Body weight and epididymal fat content on the last day of the feeding trial. Mice were given a low-fat diet (LFD, n = 8), high-fat diet (HFD, n = 8), diet containing 0.5% ASG additions to HFD (0.5% ASG, n = 8), or a diet containing 1.0% ASG additions to HFD (1.0% ASG, n = 8). Each value is shown as mean ± standard deviation. The significance test shows that ANOVA and Tukey’s multiple comparison test showed significant differences at the 5% or 1% level. *:p < 0.05, **:p < 0.01.
The epididymal fat content significantly increased in HFD (p < 0.01) and 0.5% ASG (p < 0.05) compared with LFD, with no significant differences between HFD and 1.0% ASG, whereas it significantly decreased in 0.5% ASG (p < 0.05) and 1.0% ASG (p < 0.01) compared with HFD, with no significant difference between the latter groups (Figure 1).
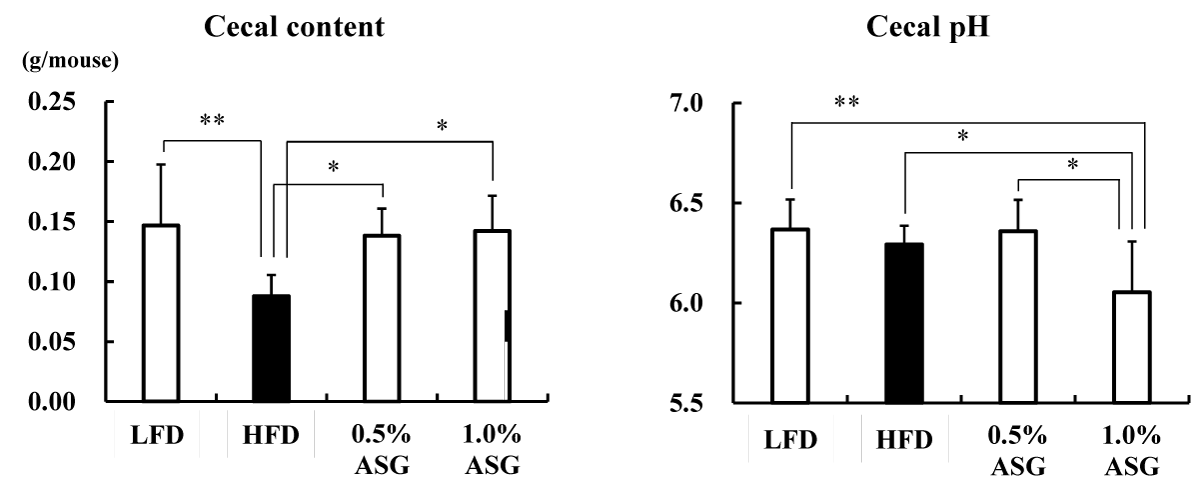
The cecum content weight was reduced in the HFD group with higher lipids in the diet compared with the LFD group and ASG group (p < 0.01) (Figure 2). In contrast, the cecum pH was significantly lower in the 1.0% ASG diet compared with the other groups (LFD vs. 1.0% ASG: p < 0.01, HFD vs. 1.0% ASG: p < 0.05, 0.5% ASG vs. 1.0% ASG: p < 0.05), although there was no significant difference between LFD and HFD.
Figure 2: Effect on cecal content and Cecal pH. Mice were given a low-fat diet (LFD, n = 8), high-fat diet (HFD, n = 8), diet containing 0.5% ASG additions to HFD (0.5% ASG, n = 8), or a diet containing 1.0% ASG additions to HFD (1.0% ASG, n = 8). Each value is shown as mean ± standard deviation. The significance test shows that ANOVA and Tukey’s multiple comparison test showed significant differences at the 5% or 1% level. *:p < 0.05, **:p < 0.01.
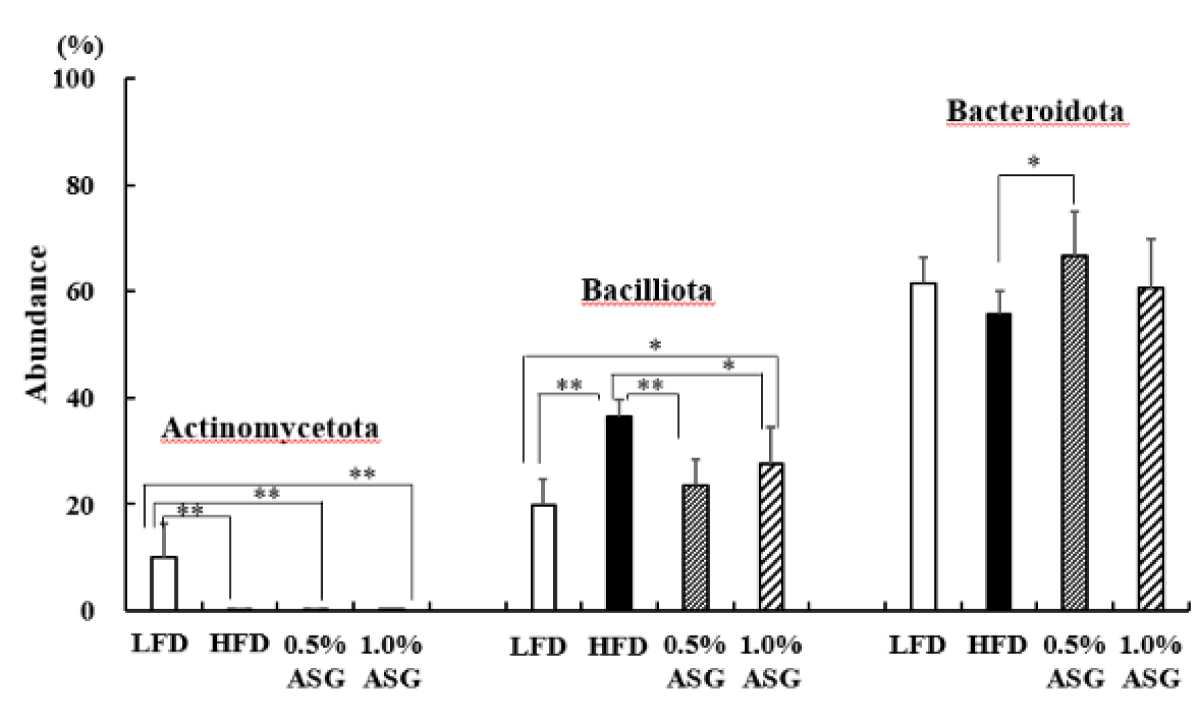
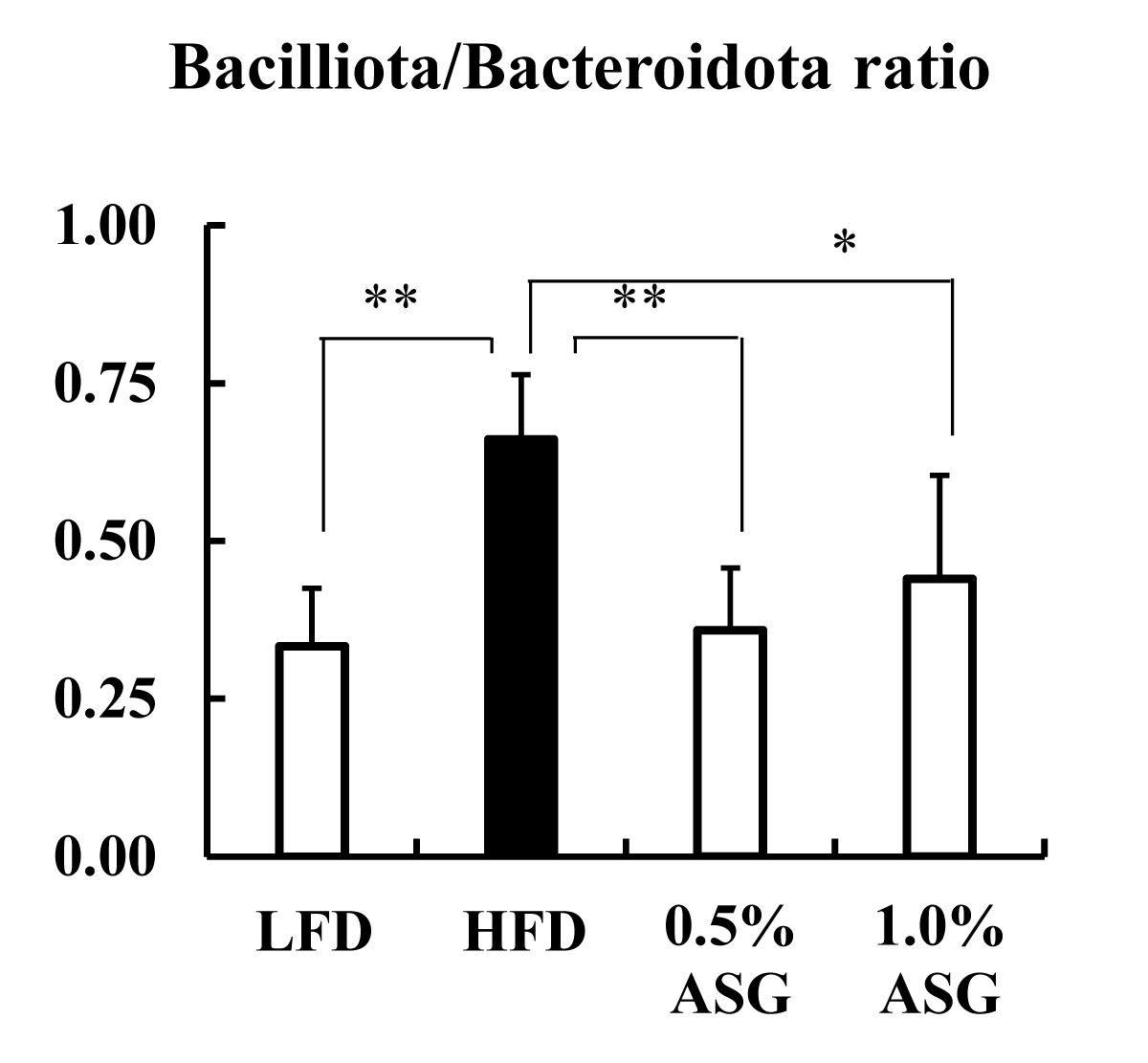
Actinomycetota was significantly lower in all other groups compared to LFD (p < 0.01) at the phylum level in the cecum. Compared to LFD and ASGs, Bacilliota (formerly known as Firmicutes) was significantly increased in HFD (LFD vs. HFD: p < 0.01, 0.5% ASG vs. HFD: p < 0.01, 1.0% ASG vs. HFD: p < 0.05) with higher concentrations of dietary lipids. In contrast, no clear differences between the diets of the organism were observed regarding the presence of Bacteroidota (formerly known as Bacteroidetes) (Figure 3). However, the ratio of Bacilliota to Bacteroidota increased in HFD (LFD vs. HFD: p < 0.01, 0.5% ASG vs. HFD: p < 0.01, 1.0% ASG vs. HFD: p < 0.05) compared to LFD and ASGs (Figure 4).
Figure 3: Effect on abundances at the phylum level in cecal content. Mice were given a low-fat diet (LFD, n = 8), high-fat diet (HFD, n = 8), diet containing 0.5% ASG additions to HFD (0.5% ASG, n = 8), or a diet containing 1.0% ASG additions to HFD (1.0% ASG, n = 8). Each value is shown as mean ± standard deviation. The significance test shows that ANOVA and Tukey’s multiple comparison test showed significant differences at the 5% or 1% level. *:p < 0.05, **:p < 0.01.
Figure 4: Effect on Bacilliota/Bacteroidota ratio in cecal content. Mice were given a low-fat diet (LFD, n = 8), high-fat diet (HFD, n = 8), diet containing 0.5% ASG additions to HFD (0.5% ASG, n = 8), or a diet containing 1.0% ASG additions to HFD (1.0% ASG, n = 8). Each value is shown as mean ± standard deviation. The significance test shows that ANOVA and Tukey’s multiple comparison test showed significant differences at the 5% or 1% level. *:p < 0.05, **:p < 0.01.
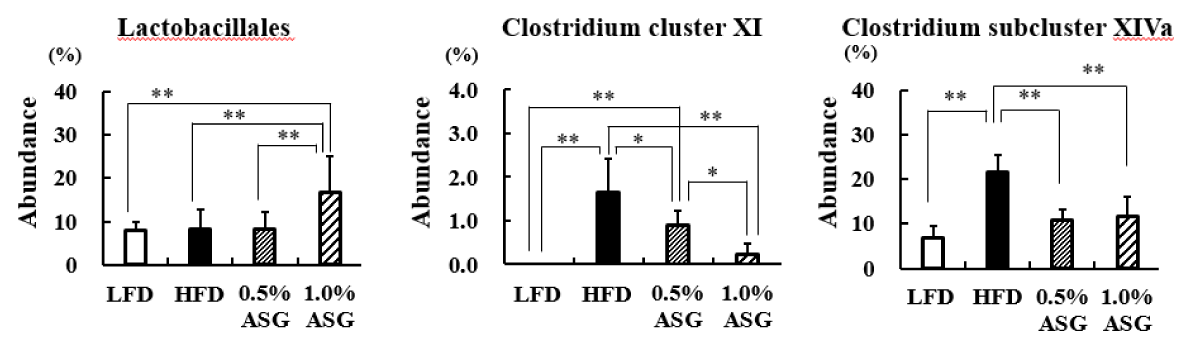
In the present study, Lactobacillales in the cecum contents significantly increased in 1.0% ASG compared to the other groups (LFD vs. 1.0% ASG: p < 0.01, HFD vs. 1.0% ASG: p < 0.01, 0.5% ASG vs. 1.0% ASG: p < 0.01). In contrast, Clostridium cluster XI (LFD vs. HFD: p < 0.01, 0.5% ASG vs. HFD: p < 0.05, 0.1% ASG vs. HFD: p < 0.01) and Clostridium subcluster XIVa (LFD vs. HFD: p < 0.01, 0.5% ASG vs. HFD: p < 0.01, 0.1% ASG vs. HFD: p < 0.01) significantly increased in HFDs but significantly decreased in ASGs compared to HFD (Figure 5).
Figure 5: Effect on intestinal bacterial flora in cecal content. Mice were given a low-fat diet (LFD, n = 8), high-fat diet (HFD, n = 8), diet containing 0.5% ASG additions to HFD (0.5% ASG, n = 8), or a diet containing 1.0% ASG additions to HFD (1.0% ASG, n = 8). Each value is shown as mean ± standard deviation. The significance test shows that ANOVA and Tukey’s multiple comparison test showed significant differences at the 5% or 1% level. *:p < 0.05, **:p < 0.01.
Even after 55 days of feeding a high-fat diet, the addition of ASG to the high-fat diet suppressed weight gain related to body fat accumulation. This suggests that ASG may have an inhibitory effect on body fat accumulation, which will be the subject of future studies.
In humans and mice, Actinomycetota, which produces short-chain fatty acids by fermentation of indigestible polysaccharides, is present in low proportions; however, the results of this study show that Actinomycetota was reduced in high-fat diets, even when ASG was added [10]. Bacilliota (formerly known as Firmicutes) dominate the bacterial flora of humans and mice followed by Bacteroidota (formerly known as Bacteroidetes) [11]. In this study, compared with LFD, the Bacilliota, which is characteristic of obese mice, was significantly elevated in HFD with higher concentrations of dietary lipids [12]. However, the addition of ASG to the HFD decreased Bacilliota. Furthermore, the ratio of Bacilliota to Bacteroidota was increased in the HFD and decreased by the addition of ASG to the diet. Ley, et al. found that two groups of beneficial bacteria, Bacteroidetes (Bacteroidota) and Firmicutes (Bacilliota), are predominant in the human gut, with obese compared with thin individuals [12].
They found that the relative proportion of Bacteroidetes (Bacteroidota) was reduced, which was shown to increase with weight loss on two different low-calorie diets. This finding suggests that gut bacteria are involved in obesity [13].
The gut microbiota is crucial in the absorption, storage, and consumption of dietary energy [14]. Furthermore, animal studies have revealed that gut microbiota regulates food intake by altering hormones that affect brain regions associated with metabolic function and eating behavior [15]. Thus, obesity and undernutrition share an important biological factor: altered composition and diversity of the gut microbiota relative to healthy humans and animals [16].
In the present study, ASG administration suppressed body weight and body fat mass accumulation in mice fed with HFD. Future research is required to determine whether this is because of the suppressive effect of ASG on body fat or the ameliorative effect of ASG on the gut microbiota through secretions.
At the order level, Lactobacillales did not differ between LFD and HFD but increased with 1% ASG intake. Lactobacillus is the largest genus of lactic acid bacteria comprising > 237 species, and several species of Lactobacillus are widely used as probiotics [17-19]. These lactobacillus and co-lactobacillus products have impressive beneficial functions, including maintenance of the epithelial barrier, antitumor effects, immunomodulation, and antagonism against pathogens [20]. In the present study, the addition of ASG to the HFD in mice increased the number of Lactobacillales in the cecum and had an ameliorative effect on the impaired energy metabolism caused by the high-fat, high-sucrose diet.
In humans, the primary bile acids are cholic acid and chenodeoxycholic acid (CDCA), which are synthesized from cholesterol. In mice, αmuricholic acid (αMCA) and βMCA are produced from CDCA in the liver. High bile salt concentrations are maintained in the duodenum, jejunum, and proximal ileum where fat digestion and absorption occur. Subsequently, bile salts are absorbed in the distal ileum by high-affinity active transport [20].
Although ileal bile salt transport is approximately 95% effective, 5% of bile salts escape the enterohepatic circulation and become substrates for important microbial biotransformation reactions in the colon [20]. Secondary bile acids produced by gut bacteria may accumulate in high concentrations in the enterohepatic circulation in some individuals and may be involved in the etiology of colon cancer, gallstones, and other gastrointestinal tract diseases. Clostridium sordellii and Clostridium scindens produce the secondary bile acid deoxycholic acid and belong to Clostridium cluster XI or Clostridium cluster XIVa, and their levels are higher in mice fed with HFD [21,22].
Therefore, we suggest that ASG intake may inhibit the conversion of bile acids to carcinogenic secondary bile acids, whose secretion is increased with the consumption of HFD.
Based on the characteristics of ASG dynamics in the intestinal tract, ASG intake inhibits the changes in the intestinal microbiota caused by HFD. This may reduce the incidence of lifestyle-related diseases caused by HFD.
We are deeply grateful to FANCL Research Institute, Ltd., for establishing the extraction and enrichment of ASG and providing ASG for this study.
- Feskens EJ, Virtanen SM, Räsänen L, Tuomilehto J, Stengård J, Pekkanen J, Nissinen A, Kromhout D. Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care. 1995 Aug;18(8):1104-12. doi: 10.2337/diacare.18.8.1104. PMID: 7587845.
- Carroll KK, Braden LM, Bell JA, Kalamegham R. Fat and cancer. Cancer. 1986 Oct 15;58(8 Suppl):1818-25. doi: 10.1002/1097-0142(19861015)58:8+<1818::aid-cncr2820581406>3.0.co;2-4. PMID: 3756806.
- Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997 Nov;42(5):776-82. doi: 10.1002/ana.410420514. PMID: 9392577.
- Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J Gastroenterol. 2016 Oct 28;22(40):8905-8909. doi: 10.3748/wjg.v22.i40.8905. PMID: 27833381; PMCID: PMC5083795.
- Grille S, Zaslawski A, Thiele S, Plat J, Warnecke D. The functions of steryl glycosides come to those who wait: Recent advances in plants, fungi, bacteria and animals. Prog Lipid Res. 2010 Jul;49(3):262-88. doi: 10.1016/j.plipres.2010.02.001. Epub 2010 Feb 6. PMID: 20138912.
- Ikeda I, Tanaka K, Sugano M, Vahouny GV, Gallo LL. Inhibition of cholesterol absorption in rats by plant sterols. J Lipid Res. 1988 Dec;29(12):1573-82. PMID: 2468730.
- Cedó L, Farràs M, Lee-Rueckert M, Escolà-Gil JC. Molecular Insights into the Mechanisms Underlying the Cholesterol- Lowering Effects of Phytosterols. Curr Med Chem. 2019;26(37):6704-6723. doi: 10.2174/0929867326666190822154701. PMID: 31438826.
- Vezza T, Canet F, de Marañón AM, Bañuls C, Rocha M, Víctor VM. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants (Basel). 2020 Dec 12;9(12):1266. doi: 10.3390/antiox9121266. PMID: 33322742; PMCID: PMC7763348.
- Nagashima K, Hisada T, Sato M, Mochizuki J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol. 2003 Feb;69(2):1251-62. doi: 10.1128/AEM.69.2.1251-1262.2003. PMID: 12571054; PMCID: PMC143637.
- Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. 2015 Nov 15;6(4):110-9. doi: 10.4291/wjgp.v6.i4.110. PMID: 26600968; PMCID: PMC4644874.
- Kobayashi R, Nagaoka K, Nishimura N, Koike S, Takahashi E, Niimi K, Murase H, Kinjo T, Tsukahara T, Inoue R. Comparison of the fecal microbiota of two monogastric herbivorous and five omnivorous mammals. Anim Sci J. 2020 Jan-Dec;91(1):e13366. doi: 10.1111/asj.13366. PMID: 32285557; PMCID: PMC7216987.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027-31. doi: 10.1038/nature05414. PMID: 17183312.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006 Dec 21;444(7122):1022-3. doi: 10.1038/4441022a. PMID: 17183309.
- Duca FA, Lam TK. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab. 2014 Sep;16 Suppl 1:68-76. doi: 10.1111/dom.12340. PMID: 25200299.
- Kairupan TS, Amitani H, Cheng KC, Runtuwene J, Asakawa A, Inui A. Role of gastrointestinal hormones in feeding behavior and obesity treatment. J Gastroenterol. 2016 Feb;51(2):93-103. doi: 10.1007/s00535-015-1118-4. Epub 2015 Sep 7. PMID: 26346735.
- Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr. 2015 Jun;34(3):341-9. doi: 10.1016/j.clnu.2014.10.003. Epub 2014 Oct 12. PMID: 25459400.
- Qin D, Ma Y, Wang Y, Hou X, Yu L. Contribution of Lactobacilli on Intestinal Mucosal Barrier and Diseases: Perspectives and Challenges of Lactobacillus casei. Life (Basel). 2022 Nov 16;12(11):1910. doi: 10.3390/life12111910. PMID: 36431045; PMCID: PMC9696601.
- Rossi F, Amadoro C, Colavita G. Members of the Lactobacillus Genus Complex (LGC) as Opportunistic Pathogens: A Review. Microorganisms. 2019 May 10;7(5):126. doi: 10.3390/microorganisms7050126. PMID: 31083452; PMCID: PMC6560513.
- Bron PA, Tomita S, Mercenier A, Kleerebezem M. Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr Opin Microbiol. 2013 Jun;16(3):262-9. doi: 10.1016/j.mib.2013.06.001. Epub 2013 Jun 28. PMID: 23810459.
- Teame T, Wang A, Xie M, Zhang Z, Yang Y, Ding Q, Gao C, Olsen RE, Ran C, Zhou Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front Nutr. 2020 Oct 22;7:570344. doi: 10.3389/fnut.2020.570344. PMID: 33195367; PMCID: PMC7642493.
- Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, Bernstein C, Holubec H, Dvorakova B, Garewal H. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007 Jan;28(1):215-22. doi: 10.1093/carcin/bgl139. Epub 2006 Aug 3. PMID: 16887864.
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006 Feb;47(2):241-59. doi: 10.1194/jlr.R500013-JLR200. Epub 2005 Nov 18. PMID: 16299351.