Research Article
Micronutrient deficiency, a novel nutritional risk factor for insulin resistance and Syndrom X

Christopher Edet Ekpenyong*
Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria
*Address for Correspondence: Dr. Christopher Edet Ekpenyong, Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria, Tel: +234823347719; Email: [email protected]; [email protected]
Dates: Submitted: 23 November 2018; Approved: 29 November 2018; Published: 30 November 2018
How to cite this article: Ekpenyong CE. Micronutrient deficiency, a novel nutritional risk factor for insulin resistance and Syndrom X. Arch Food Nutr Sci. 2018; 2: 016-030. DOI: 10.29328/journal.afns.1001013
Copyright License: © 2018 Christopher Edet Ekpenyong. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Minerals; Vitamins; Deficiency state; Hyper-insulinemia; Syndrome X
Abstract
Emerging evidence indicates that micronutrient deficiency could play a significant role in the pathogenesis and progression of many chronic diseases including diabetes mellitus, hypertension, obesity, dyslipidemia, hyperuricemia, kidney disease, cancer, anemia and other cardio-metabolic and neurodegenerative diseases through the induction of Insulin resistance (IR). However, there are still gaps in our scientific knowledge regarding the links between micronutrient deficiencies, IR, and cardio metabolic disorders. This review provides current information on recent advances and a global perspective regarding the relationship between micronutrient deficiency, IR, and cardio metabolic disorders. Empirical evidence indicates that deficiencies in either micronutrients associated with insulin activity (such as Chromium, manganese, magnesium, and iron) or antioxidant enzyme cofactors (such as vitamin A, copper, zinc, and manganese) could impact several physiological processes leading to a cascade of metabolic and biochemical derangements such as B-cell apoptosis, loss of islet cell mass, defective tyrosine kinase activity, oxidative stress, pancreatic β-cell dysfunction, reduction in lean body mass, defective insulin signaling mechanism, elevated protein kinase C activity, and excess intracellular calcium. Collaboratively, these states of metabolic malfunctioning are associated with IR, which triggers the onset of many cardio metabolic diseases. Undoubtedly, the prevention of micronutrient deficiency may indeed ameliorate the incidence of IR and cardio-metabolic disorders in those at risk and in the general population.
Introduction
Insulin resistance syndrome is a worldwide metabolic disorder with increasing trend among all races. Insulin resistance (IR) describes a state of non-responsiveness of the insulin-responsive tissues such as the liver, muscles and fats to the effect of insulin signals [1,2], leading to hyperglycemia.
It depicts a condition in which a given concentration of insulin produces a less-than expected biological effect. Specifically, IR has been defined as the requirement of 200 or more units of insulin per day to attain glycemic control and prevents associated complications [3].
IR is a state of metabolic dysfunction that orchestrates a cluster of metabolic disorders such as diabetes mellitus, hypertension, hyper-uricemia, obesity and dyslipidemia [4,5]. These syndrome clusters are otherwise known as syndrome X or the dysmetabolic syndrome.
Several variables are used for predicting insulin non-responsiveness (in-sensitivity), both in research and clinical practice. The euglycemic insulin clamp and the intravenous glucose tolerance test (IVGTT) are standard research methods for determining insulin sensitivity, while the fasting insulin, glucose homeostatic model assessment (HOMA), insulin-to-glucose ratio and several individual variables (e.g., body mass index (BMI), blood pressure (BP), waist and hip circumference, fasting triglycerides, high-density lipoprotein cholesterol (HDL-C), glucose, insulin, hepatic enzymes determinations) are more feasible assessment methods in clinical practice and population based studies [5].
However, it should be noted that the predictive value of these variables are more sensitive when used in combination than a single variable.
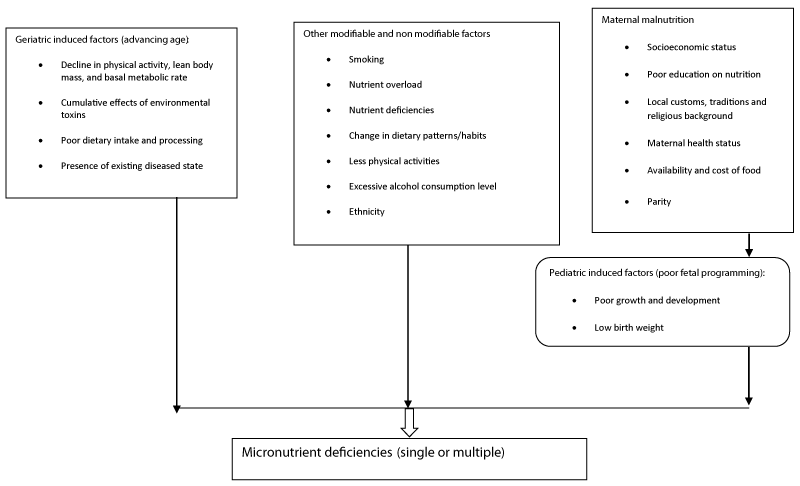
The etiology of IR is complex and multi-dimensional and can be classified into genetic and acquired. Acquired causes include medications (steroids and anti-retroviral therapy (protease inhibitors)), life style factors (poor dietary habits and physical inactivity), physiological (pregnancy), and some diseases such as hepatitis C and polycystic ovarian disease, aging and hyperglycemia. From dietary perspective, several diets or dietary factors have been implicated in the development of a state of IR [6], and in particular micronutrients deficiency. IR steming from micronutrients deficiencies is a well-recognized metabolic disturbance that is at the root cause of diseases and maladies of cardio-metabolic disorders. Micronutrients consist of vitamins and minerals which are required in small amounts to enhance normal physiological and biochemical processes in the body. They include, but are not limited to, vitamins A, B, C, D and E, calcium, folate, iodine, iron, zinc, chromium, biotin, thiamine, magnesium and selenium as well as many others [7]. They are classified as micronutrients not by virtue of functional importance but by virtue of the amount required by the body. Although they are required in trace concentrations in the body, they are essential in triggering the thousands of chemical reactions essential to life and maintenance of good health including effects on insulin production and activities. MNDs or excesses usually lead to human disease. Empirical evidence indicates that deficiencies in either micronutrients associated with insulin production or activities could impact several physiological and biochemical processes leading to a cascade of metabolic and biochemical derangements including oxidative stress , pancreatic β-cell dysfunction, defective tyrosine kinase activity, reduction in lean body mass, defective insulin signaling mechanism, elevated protein kinase (PKC) activity, and excess intracellular calcium as shown in figures 1,2. Different factors can cause micronutrient deficiency, which can be categorized into 1) poor maternal/pediatric nutrition (resulting from poor fetal programming), 2) other modifiable/non-modifiable factors that a prone individual is exposed to in the environment, and 3) other geriatric factors caused by advancing age (Figure 3). These factors act either individually or in association with other factors to culminate in either single-or multiple-micronutrient deficiencies. The single-micronutrient deficiency states are comparatively recognizable and treated, whereas multiple-micronutrient deficiency is difficult to diagnose and manage [8].
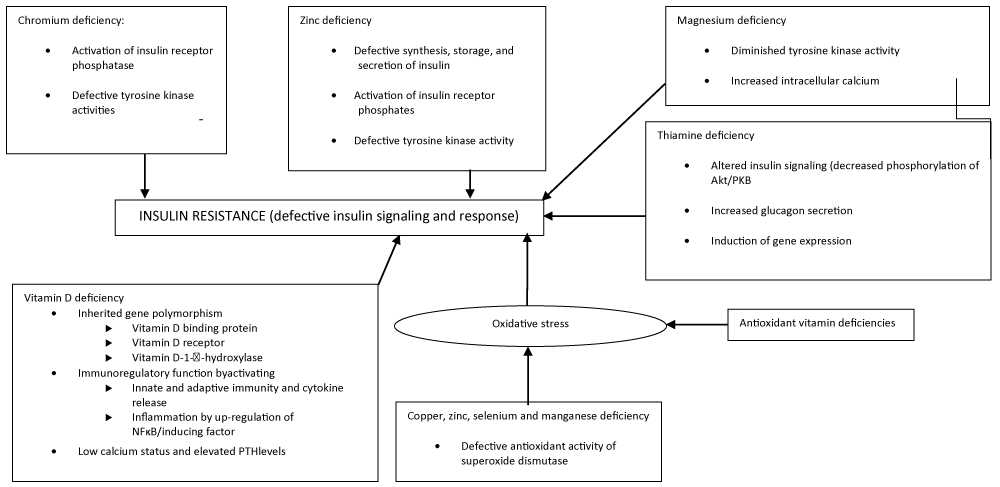
Figure 1: The link between different micronutrient deficiencies and IR. NF-B, nuclear factor-B; PKB, protein kinase B; PTH, parathyroid hormone.
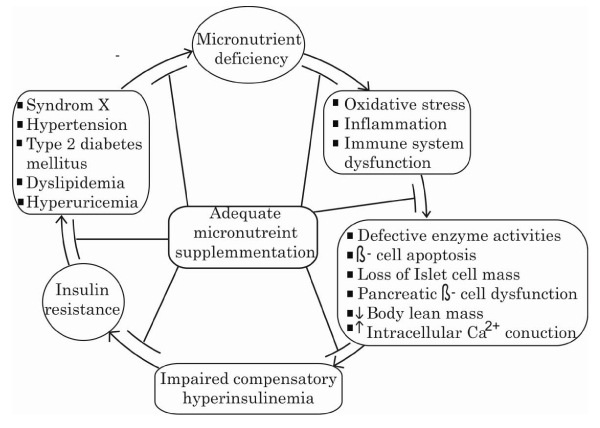
Figure 2: A summary of the possible associated mechanisms linking micronutrient deficiencies with IR and the MetS.
The diverse etiology of IR makes it difficult to elucidate, and by extension difficulty to choose appropriate therapeutic strategy. This has partly culminated in the treatment failure recorded in some clinical studies such as the ACCORD trail [9], in which several hypoglycemic agents including insulin injection failed to achieve normoglycemia.
The increasing evidence that micronutrient deficiencies (MND) could play a significant role in the pathogenesis and progression of IR and dysmetabolic syndrome clusters, and the recent studies indicating that diets (fruits and vegetables) rich in micronutrient and antioxidants or supplementation with MN could ameliorate these disorders suggest adequate MN intake could be a novel nutritional/therapeutic target for IR and dysmetabolic syndrome clusters.
However, there is still a lack in understanding the mechanistic links between MND and IR. More so, results of studies examining the role of MND in IR are conflicting.
In this review work, we analyzed pooled population based data on the relationship between MND and IR, and extend the discussion on the plausible links with dysmetabolic disorders.
Methods
Literature search
A literature search was conducted using Google Scholar and PubMed Search engines, to identify English language articles published up to 2017 that explored the relationship between micronutrient deficiencies and insulin resistance using keywords such as insulin resistance, minerals, vitamins, magnesium, chromium and, zinc. For each article selected, we considered the effect of the mineral or vitamin on insulin activity, and possible mechanism of action such as vitamin or mineral-deficiency-induced defective insulin signaling mechanisms, defective glucose sensing apparatus induction of hyper-insulinemia, immune system dysfunction, oxidative stress, inflammatory reactions, pancreatic β-cell dysfunction and elevated protein kinase activity.
In all one hundred and twenty-five articles were selected and after applying the inclusion criteria, only eighty five articles were included in the review. Exclusion criteria included articles with incomplete data, methodology flaw, inappropriate analytical methods and unadjusted confounding variables.
Micronutrient deficiency and insulin resistance
Magnesium (Mg2+) as an essential micronutrient abounds in significant amounts in living cells with its plasma concentration being remarkably consistent in healthy subjects [10]. It is a cofactor of many enzymes involved in glucose metabolism, especially those using high-energy phosphate bonds [11]. Mg2+ deficiencies provoke a cascade of many biochemical and symptomatic alterations such as in T2DM, hypertension, and other cardio metabolic diseases. It has also been reported that patients with hypomagnesemia can present with ischemic cardiac insufficiencies, vascular complications of DM, and hypertension [12,13]. Additionally, hormonal, neurological, gastrointestinal, renal, and muscular dysfunction has been associated with hypomagnesemia [12,14,15].
Plasma and [Mg2+]i are tightly regulated by several factors including insulin [10,16]. A number of in vitro and in vivo studies have demonstrated that insulin modulates the mobilization of Mg2+ from the extracellular to intracellular space [10,11,17]. Insulin also regulates the [Mg2+]i by stimulating erythrocyte Mg2+ uptake and forming a complex with the plasma membrane adenosine triphosphatase pump [17]. Hence, Mg2+ is an essential cofactor in all adenosine triphosphate transfer reactions and plays a critical role in the phosphorylation of the insulin receptor [18]. Aside from insulin, Mg2+ regulates the entrance and exit of calcium (Ca2+) in cells. Mg2+ has been described as nature’s physiological Ca2+ blocker [19]. Intracellular Ca2+ has been associated with insulin-induced action. Begum et al. [20], showed that high intracellular Ca2+ inhibits insulin receptor dephosphorylation in adipocytes. Thus, in skeletal muscle and fat tissues, IR could be expected in the presence of increased Ca2+ and suppressed Mg2+. In hypertension, diabetes, and obesity, a deficiency in the ([Mg2+]i) is correlated with an excess of intracellular calcium [10], causing further activation of protein kinase C (PKC), which is a constitutive regulator of the insulin receptor [21]. There is significant evidence that IR is also induced by elevated PKC activity [22].
By stimulating Ca2+-dependent potassium (K+) channels, intracellular Mg2+ has been shown to be effective in modulating insulin action (mainly oxidative glucose metabolism), offsetting calcium-related excitation-contraction coupling, and decreasing smooth muscle cell responsiveness to depolarizing stimuli [10]. Mg2+ may exert a potent inhibition on Ca2+ channel activity and interact with Ca2+, which secondarily mediates insulin action. It is possible that the association between a low [Mg2+]i and IR is not primary but is related to abnormalities of other cations, such as Ca2+ [23].
Intracellular Mg2+ may play the role of second messenger for insulin action, contributing to IR. Further studies revealed that a decrease in the [Mg2+]i is associated with attenuation of insulin’s ability to stimulate glucose uptake in insulin-sensitive tissues such as adipose cells and skeletal muscle tissues [24]. Different studies have related insulin receptor binding with tyrosine kinase activity and IR [18,25,26]. In fact, in their studies, Suâre et al. [27], observed that IR in magnesium-deficient rats might be attributed to the defective tyrosine kinase activity of the insulin receptor. Also, Paolisso and Barbagallo [10], hypothesized that the low availability of intracellular Mg2+ diminishes the tyrosine kinase activity and increases the vascular constriction mediated by Ca2+, impeding the relaxation of cardiac and smooth muscles; thereby interfering in cellular glucose utilization. This was postulated as the underlying mechanism by which Mg2+ deficiency contributes to the raised blood pressure and peripheral IR in hypertension and T2DM. The interaction between low [Mg2+]i with intracellular Ca2+ and other components in IR is illustrated in figure 1.
Manganese deficiency and insulin resistance
Manganese (Mn) is an essential trace element that participates in several metabolic activities in the body including carbohydrate, fat and protein metabolism. Common sources of Mn include whole grains, nuts, dried legumes and pineapple. It acts as a cofactor for several enzymatic systems, and is a key component of the manganese - superoxide – dismutase (MnSOD) complex. MnSOD is localized in the mitochondrial matrix. The anti-oxidative effect of MnSOD protects the mitochondrial matrix from destruction by the ROS. Mn is also involved in normal immune response and is required in normal insulin synthesis and secretion [28]. Several studies have reported inverse association of serum Mn level with insulin activity and hence blood sugar level [29-32], in several countries. Interestingly, in some of these studies age and sex tend to modify the association between serum Mn levels and insulin sensitivity. In a Spanish study, higher intake of Mn correlated with improved insulin sensitivity and reduced risk of developing diabetes mellitus [32]. The underlying mechanisms through which Mn improves insulin sensitivity include improvement in insulin secretion, decreased lipid peroxidation and improved mitochondrial function [33,34].
Chromium deficiency and insulin resistance
Chromium (Cr) is an essential cofactor required for optimal insulin signaling. It exerts beneficial roles in the regulation of insulin action, the MetS, and cardiovascular diseases [35]. The main sources of Cr are food, stainless steel pots used in cooking, and intracellular stores in the liver. Different clinical trials and systemic reviews have been conducted to assess the effect of Cr supplementation on insulin sensitivity and resistance [36,37]. This review focuses on the molecular mechanisms associated with Cr deficiency and IR.
Studies have shown that Cr-bound to the oligopeptide chromoudulin enhances the tyrosine kinase activity of the insulin receptor and inhibits phosphotyrosine phosphatase activity, and hence amplifies the intracellular insulin signal pathway [38-40].
Insulin binds to the α-subunit of the insulin receptor, bringing about conformational changes leading to auto-phosphorylation of the β-subunit of the insulin receptor. In response to increased blood sugar, insulin levels rise and Cr is mobilized from the blood to the insulin-dependent cells [36,41,42], which is facilitated by transferrin. There is also a transfer of Cr-bound to transferrin to apochromodulin, the low-molecular-weight Cr-binding substance [42]. Apochromodulin, when it is fully activated, is able to increase the activity of insulin receptor kinase and inhibit that of insulin receptor phosphatase. The activation of insulin receptor kinase activity by Cr and the inhibition of insulin receptor tyrosine phosphatase are responsible for the increased phosphorylation of the insulin receptor, which is associated with increased insulin sensitivity [43]. In the Cr-deficient state, this established correlation could be reversed or even blocked, a condition contributing to IR in the long term (Figure 1).
Selenium deficiency and IR
Selenium (Se) is an essential mineral found in foods such as fish and whole grain cereals as organic selenocysteine or selenomethionine.
Selenium occupies the active site of glutathione peroxidase (GPx) [44,45], and potentiates cellular response to oxidative stress [46]. It aligns with other cellular antioxidants to protect bio-membrane against oxidative damage. Normal reference value for adults is 70 to 150ng/ml (0.15part per million (ppm)).
Normal daily intake of Se is 0.01 to 0.04ppm. However, regional variation in daily dietary intake of Se has been reported due to differences in ethnic diet. Concentration of serum Se<40ng/ml is associated with loss of glutathione peroxidase (GPx) activity.
Conflicting Epidemiological data on the relationship between body Se status, IR and glucose metabolism, have been reported.
Studies of both human and experimental animals indicate that adequate intake of Se (within the limit of nutrient requirement) protects against IR and type2 diabetes mellitus by mimicking insulin function. Conversely, low body status of Se is associated with atrophy of pancreas [47], impaired insulin-stimulated glucose oxidation in adipocytes [48], decrease islet cell function, low insulin secretory reserve and low free radical scavenging system [49]. There are also associated abnormality in glucose and lipid metabolism [50]. Likewise, high intake of Se has been reported to be associated with metabolic aberrations similar to those observed in low Se intake. For instance, a study by Zhang et al. [51] showed that animals fed 3.0ng/kg of dietary Se developed hyper-insulinemia, IR and glucose intolerance. Another study showed that animals fed diet supplemented with 0.4ng/kg of Se developed hyper-insulinemia and loss of insulin sensitivity compared with control animals [52]. Abnormal serum Se levels perse may not in all cases be responsible for afore mentioned metabolic aberrations. Deficiency in some Se proteins has also been implicated in IR. Studies have shown that although circulating Se status is adequate, deficiency of Se-cystiene lyase (Scly) can produce metabolic aberrations similar to those associated with abnormal serum Se levels [53]. Also, high body Se status has been reported to cause a dose-dependent increase in GPxl mRNA or GPxl activity in pancrease, liver and erythrocytes of dams and Sels mRNA levels in the liver of the offspring, and leading to hyperuricemia and IR in the high Se-diet group [51]. Over expression of some Se proteins such as cytosolic GPx1 and selenoprotein I (SEPPI) have been implicated in IR. Gpx1 regulates energy metabolism, insulin synthesis, secretion and function.
Down-regulation of various genes involved in the pathways of insulin signaling and glucose sensing activities is the postulated pathophysiologic mechanism under lying the high Se intake-induced impaired glucose sensing and defective insulin secretion and type 2 diabetes mellitus [54].
Zeng et al. [54] reported increased activities of GPx in rat fed high Se diet compared to those fed low Se-diet. Evidently, intake of dietary Se within the limit of nutritional requirement protects against hyperinsulinamia, IR and glucose intolerance.
Zinc deficiency and insulin resistance
Zinc (Zn2+) is another essential micronutrient that plays a contributory role in maintaining cellular homeostasis owing to its influence on different biological processes. Zn2+ deficiency or invariably dysfunctional Zn2+signaling has been documented to be associated with a wide array of diseases including cancer, autoimmune diseases, cardiovascular diseases, neurodegenerative diseases, and diabetes [43]. It is reported that Zn2+ plays a key role in the synthesis, secretion, and action of insulin [43]. Zn2+ availability and delivery to cells and tissues are tightly regulated by a family of proteins known as Zn2+ transporters [55-58]. Several studies investigating the mechanisms of the insulin-mimetic activity of Zn2+on glucose and lipid metabolism [59-62] have concluded that Zn2+ plays a dynamic role as a cellular second messenger in the control of insulin signaling and glucose homeostasis. Zn2+ is said to mediate its insulin-mimetic effect in part through the inhibition of protein tyrosine phosphates, which increases the net phosphorylation of the insulin receptor and activates the signaling cascade [62,63]. Zn2+ also induces the stimulation of glucose uptake and lipogenesis in adipocytes [60], tyrosine phosphorylation of insulin, insulin-like growth factor 1 receptor, and insulin receptor substrate-1 (IRS-1) [62,64], activation of mitogen-activated protein kinesis (MAPKs) including extracellular signal regulated kinesis 1 and 2 and-Jun N-terminal kinas (JNK); and glycogen synthesis through the inhibition of glycogen syntheses kinase-3 [61]. Zn2+ therefore does not affect the action of insulin on insulin receptors, but its action is post receptor either through activation of receptor tyrosine kinase phosphorylation or activation of the phosphatidylinositol 3-kinase/Akt pathway leading to the activation of glucose transporter 4, which increases glucose mobilization into cells [65-67].
The inhibition of protein tyrosine phosphatase by Zn2+ under physiological conditions involving a Zn2+ transport mechanism has widespread implications for the understanding of IR and disease progression [43].
Vitamin A deficiency and IR
Vitamin A (VA) (retinoids) exists in plant and animal foods. Its functions include maintenance of vision, immunity and embryogenesis [68]. It is also involved in the development of pancrease and insulin producing β-cells [69,70].
Recent evidence indicates that VA regulates metabolic pathways central to the pathogenesis of metabolic syndrome clusters including obesity [71], diabetes mellitus [72], dyslipidemia [73] and cardiovascular disease [7]. Central to the metabolic pathways is the improvement in β-cell mass and function, hence insulin secretion and responses. VA status has been found to influence several biomarkers of insulin sensitivity such as obesity, triglyceride and insulin levels [51]. Interestingly, in one study, VA deprivation had no effect on insulin sensitivity [74]. One major role of VA is to maintain pancreatic β-cell mass and function in adults [74], whereas during fetal life, VA maintains the endocrine function of the pancreatic β-cell [75,76].
VA deficiency (VAD) has been reported to cause marked, but dose dependent histomorphological and biochemical changes in the pancrease, including significant β-cells apoptsis, loss of β-cells mass, decrease pancreatic Islet area, proliferation of glucagon producing α-cells and reduction in basal pancreatic insulin levels [74]. There is also a reduction in pancreatic transcript levels of major intracellular carrier and metabolizing proteins including pancreatic cellular crbpl and cyp26a1 expression.
VA supplementation reversed most of these changes and protects against IR [77,78]. However, higher serum levels of VA have been found to increase the risk of diabetes mellitus in some other studies [79], probably due to the effect of unadjusted covariates.
Literature evidence suggests that VA and other related synthetic retinoids have the potential for use in management of dysmetabolic disorders. However, more studies are needed to determine efficacy and safety.
Folate deficiency and IR
Folate is a water soluble vitamin found in a wide variety of plant and animal foods including yeast, green vegetables, eggs, liver, kidney and meats.
It is a source of one carbon group for deoxyribonucleic acid methylation and involves in homocysteine metabolism to methionine. Deficiency of folate causes hyper-homocysteinemia which has an inverse relationship with IR and T2DM. Low folate diet is associated with impaired methylation of genes associated with DM including impaired methylation of NKX2-2, UTS2 and CYP2 el al. [80]. The inverse relationship between serum folate concentration and IR have been extensively documented [81].
In a double blind, parallel, identical placebo-drug, randomized study among 50 patients with metabolic syndrome, Setola et al. [82] found that prolonged folate treatment significantly improved markers of IR (insulin levels and HOMA), endothelial dysfunction and caused a reduction in serum homocysteine level. Interestingly, positive, significant relationship between the reduction in homocysteine and insulin level was observed in that study, although the study failed to establish the direction of association.
Low meternal folate caused hyperinsulinemia and a reduction in adiponectin/leptin ratio (marker of insulin sensitivity) in offspring [28], and vice versa.
In a recent cross-sectional study of 1530 US non-diabetic adults Li et al. [83], used multiple linear regression models to demonstrate a significant inverse relationship between serum folate level and markers of IR independent of several potential confounding factors.
Induction of hyperhomocysteinemia is the postulated pathophysiologic mechanism underlying folate deficiency induced IR and T2DM. Homocysteine in its active form (Homocysteine thiolactone) which is known to cause IR by inhibiting the insulin-stimulated tyrosine phosphorylation of insulin receptor β-subunit and its substrates insulin receptor substrate-I and P60-70. Also, homocysteine thiolactone has been found to decrease P85 regulatory subunit of phosphatidylinositol 3-kinase activity, with a resultant decrease in insulin stimulated glycogen synthesis [82,84].
Biotin and thiamine deficiencies and insulin resistance
Biotin regulates transcription of the insulin receptor and improves β cell function [85,86]. Larrieta et al. [85] discovered that biotin deficiency in experimental animals caused a disruption of islet morphology and an increase in the number of α-cells in the islet core. There was also impairment in glucose and insulin tolerance, indicating defective insulin sensitivity/signaling. The altered insulin signaling was linked to a decrease in phosphorylated Akt/PKB but not to any change in insulin receptor transcription. It is believed therefore that biotin deficiency promotes hyperglycemic mechanisms such as increased glucagon concentration, decreased insulin sensitivity, and secretion and induction of hexokinase gene expression [85].
Thiamine as an essential micronutrient acts as a cofactor for several enzymes involved in glucose and amino acid metabolism, especially pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and α-keto acid decarboxylase, which all serve as regulatory enzymes mediating glycolysis, citric acid, and pentose phosphate pathways, respectively [87].
Vitamin C deficiency and insulin resistance
Ascorbic acid (vitamin C) is water soluble and an antioxidant vitamin that is involved in many enzymatic reactions including collagen synthesis. Deficiency of vitamin C results in scurvy, a disease characterized by defective formation of collagen in the skin, bone, cartilage, dentine and blood vessels [88]. Structurally, vitamin C is similar to glucose; hence compete with glucose for transport into the Islet cells [88]. However, the role of vitamin C on insulin sensitivity has been controversially discussed.
In a randomized controlled study among type 2 diabetes patients, Mason et al. [93] reported that Vitamin C supplementation significantly increased peripheral insulin sensitivity through improvement in skeletal muscle oxidation activity and insulin-during hyper-insulinemia, but without significant alteration in basal oxidative stress markers, endogenous glucose production and glycosylated hemoglobin.
This study findings conflict with several other study findings in the literature. For instance, Khodaeian et al. [89] in a meta-regression test reported that vitamin C and vitamin E either solely or in combination with other antioxidants failed to improve insulin sensitivity in diabetic patients. In 2015, Picklo and Thyfault [90], demonstrated that supplementation of vitamin C and E failed to modify exercise-induced improvement in insulin sensitivity in obese rodents. These findings provide additional support to a previous study findings by Johnston and Yen [91], who reported in a double-blind, plaebo-controlled study among normoglycemic subjects that intake of mega doses of vitamin C (2gm) for 2 weeks caused a delay in insulin release and hence prolonged post-prandial hyperglycemia during an oral glucose challenged test.
In a recent systematic review of 22 randomized controlled trails, Ashor et al. 2017 [92] found that vitamin C did not modify circulating biomarkers of glycaemia and insulin regulation including glucose, HbA1C and insulin concentrations. However, age modified the effect of vitamin C on insulin concentration, although the effect size was confounded by body mass index, plasma glucose levels and the duration of the intervention. Nevertheless, individualized intervention may prove more beneficial in the relationship between vitamin C supplementation and IR. More studies are still needed to evaluate the effect of individual characteristics and duration of supplementation on the relationship between vitamin C and IR. However, intake of a balance diet may represent a feasible strategy to modify the heterogeneity among studies. Since human are unable to produce vitamin C-due to the absence of L-gulonolactone an enzyme required to catalyze the final step in the synthesis of vitamin C [93,94].
Vitamin D deficiency and insulin resistance
When exposed to sunlight, 7-dehydrocholesterol in the skin is rapidly converted to pre-vitamin D3, which is slowly converted to vitamin D3. Vitamin D can also be obtained from dietary vitamins D2 and D3 incorporated into chylomicrons; hence, vitamin D3 is also ingested in the diet. Vitamin D in the circulation is bound to a globulin vitamin D-binding protein, which transports it to the liver where it is converted to 25-hydroxyvitamin D by vitamin D-25-hydroxylase. The biologically inactive 25-hydroxyvitamin D is then converted in the kidneys to active 1, 25-dihydroxy vitamin D by 25-hydroxyvitamin D3-1α-hydroxylase (1-OHase). Increased serum levels of parathyroid hormone, sex hormones, calcitonin, and prolactin, in conjunction with a low phosphorus/calcium ratio; all act to increase renal 1, 25-dihydroxyvitamin D production. However, fibroblast growth factor 23 and 1, 25-dihydroxyvitamin D has feedback functions to inhibit 1-OHase. Finally, the active 1,25-dihydroxyvitamin D can bind to a vitamin D receptor (VDR)-retinoic acid X receptor (RXR) complex in the intestine, bone, and parathyroid glands and then exert the classical function of mineral homeostasis [95]. In addition, it also has no skeletal functions when bound to VDR-RXR in other organs (breast, colon, prostate, kidney, and pancreas) or immune cells (macrophages/monocytes) [40].
It has been proposed that vitamin D deficiency plays an important role in IR. The potential role of vitamin D deficiency in IR has been proposed to be associated with inherited gene polymorphisms in vitamin D-binding protein, VDR, and vitamin D 1α-hydroxylase genes. Other roles have been proposed to involve immuno regulatory functions by activating innate and adaptive immunity and cytokine release, activating inflammation by up-regulation of nuclear factor κB (NFκB) and inducing tumor necrosis factor α, and other molecular actions to maintain glucose homeostasis and mediate insulin sensitivity by a low Ca2+ status or obesity or by elevating serum levels of parathyroid hormone [40,96-101].
Via different mechanisms, 1, 25-dihydroxyvitamin D plays an essential role in glucose homeostasis. In fact, 1, 25-dihydroxyvitamin D improves the insulin sensitivity of its target cells (liver, skeletal muscle, and adipose tissue) and also improves β-cell function. In addition, 1,25-dihydroxyvitamin D protects β-cells from detrimental immune attacks, directly by its action on β-cells as well as indirectly by acting on different immune cells, including inflammatory macrophages, dendritic cells, and a variety of T cells. Macrophages, dendritic cells, T lymphocytes, and B lymphocytes are also known to synthesize 1,25-dihydroxyvitamin D, all contributing to the regulation of local immune responses [40,102]. Evidently, these effects of vitamin D deficiency, acting either in concert or alone, all serve in one way or another to increase IR (Figure 2).
Vitamin E and Insulin resistance
Vitamin E is another antioxidant vitamin which has been extensively investigated for its ability to improve insulin sensitivity and oxidative stress. For instance, in a randomized double blind placebo controlled trial among 70 metabolic syndrome patients, age 29-57 years, Shidfar et al. [103], used 400mg of vitamin E for 3 months to achieve a significant improvement in insulin sensitivity and other markers of insulin resistance including significant reduction in blood pressure, serum glucose and TG levels. These results were consistent with previous studies with similar findings.
In 2006, Moorthi et al. [104] found that pre-treatment with vitamin E prevented hydrogen peroxide-induced impairment in insulin action through its improvement in free radical defense system.
In one study, administration of 800 IU vitamin E for 3 months improved insulin sensitivity and associated features of IR in overweight individuals [105]. At variance with the afore studies, Sanchez-bugo et al. [106] in a cross-sectional study among African American, Hispanic and non-Hispanic white men and women reported that supplementation with vitamin E and C failed to bring about improvement in insulin sensitivity, which could have been due to the confounding effects of several covariates. In several of the studies showing direct correlation between vitamin E supplementation and improvement in insulin sensitivity, vitamin E was found to exhibit both antioxidant and non-antioxidant effects. For instance, vitamin E maintains insulin cellular sensitivity by inhibiting the activation of protein kinase C which is known to decrease the sensitivity of insulin receptor. By increasing the sensitivity of insulin receptor, cellular insulin is improved. Vitamin E is posited to increase the activities of diacylglycerol kinase (DAGK) an enzyme known to degrade diacylglycerol (DAG). DAG is an activator of an intracellular signal molecule (PKC) which is known to desensitize insulin receptor by phosphorylating threonine or serine residues on the receptor and leading to alteration in conformity of the receptor.
Vitamin E is also posited to directly alter the conformity of the cell membrane thereby making it unsuitable for the activity of PKC or DAG.
Conclusion
Evidently, it is empirical to state that the possible link between micronutrient deficiency and MetS clusters, namely dyslipidemia, hypertension, hyper-uricemia, T2DM, obesity, and a prothrombotic and pro-inflammatory state, is through the IR path.
Unmistakably, a deficiency in these different micronutrients (especially those associated with insulin action) has been shown to be a possible catalyst in the reaction pathway in the etiology of most of the MetS clusters.
Collaboratively, it could be postulated, therefore, that the link between micronutrient deficiency and the MetS clusters is a deficit in insulin action due to oxidative stress or a deficit in the activities of insulin-associated enzymes, which, over time, if not managed, could result in IR. This sets the pace for other components of the clusters to gradually manifest.
References
- Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. Adolescents. Diabetes care. 2006; 29: 2427-2432. Ref.: https://goo.gl/Mi9QTy
- Do, HD, Lohsoonthorn V, Jiamjarasrangsi W, Lertmaharit S, Williams MA. Prevalence of Insulin resistance and its relationship with cardiovascular disease risk factors among Thai adults over 35 years old. Diabetes Res Clin Pract. 2010; 89: 303-308. Ref.: https://goo.gl/y4DyPi
- Agus ZS. Hypomagnesemia. J Am Soc Nephrol. 1999; 10: 1616-1622. Ref.: https://goo.gl/pNXTZ2
- Defronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991; 14: 173-194. Ref.: https://goo.gl/FLtAvz
- Mcauley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, et al. Diagnosing insulin resistance in the general population. Diabetic care. 2001; 24: 460-464. Ref.: https://goo.gl/UjF2TN
- Storlien LH, Higgins JA, Thomas TC, Brown MA, Wang HQ, et al. Diet composition and models. Br J Nutr. 2000; 83, suppl 1: S85-S90. Ref.: https://goo.gl/2tc558
- Ekpenyong CE. Micronutrient vitamin deficiencies and cardiovascular disease risk. Advancing current understanding European current understanding. European Journal of Preventive Medicine. 2017; 5: 1-18. Ref.: https://goo.gl/RsBHgF
- Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets. 2007; 7: 65-74. Ref.: https://goo.gl/7zgC4X
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, et al. Effect of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545-2559. Ref.: https://goo.gl/ZCv3JB
- Sone H, Ito M, Sugiyama K, Ohneda M, Maebashi M, et al. Biotin enhances glucose stimulated insulin secretion in the isolated perfused pancreas of the rat. J Nutri Biochem. 1999; 10: 237-243. Ref.: https://goo.gl/6u9WYJ
- Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002; 106: 286-288. Ref.: https://goo.gl/meyQKm
- Abate N, Vega GL, Garg A, Grundy SM. Abnormal cholesterol distribution among lipoprotein fractions in normolipidemic patients with mild NIDDM. Atherosclerosis. 1995; 118: 111-122. Ref.: https://goo.gl/UohBtd
- Laakso M, Sarlund H, Mykkanen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis. 1990; 10: 223-231. Ref.: https://goo.gl/HiwAux
- Eckel RH, Prasad PA, Kern PA, Marshall S. Insulin regulation of lipoprotein lipase in cultured isolated rats adipocytes. Endocrinology. 1984; 114: 1665-1671. Ref.: https://goo.gl/xYjGWc
- Arai T, Yamahita S, Hirano K, Sakai N, Kotani K, et al. Increased plasma cholestryl ester transfer protein in obese subjects: a possible mechanism for the reduction of serum HDL cholesterol levels in obesity. Arterioscler Thromb. 1994; 14: 1129-1136. Ref.: https://goo.gl/758TrB
- Dullart RP, Sluiter WJ, Dikkeschei LD, Hoogenberg K, Van Tol A. Effects of adiposity on plasma lipid transfer protein activities: a possible link between insulin resistance and high density lipoprotein metabolism. Eur J Clin Invest. 1994; 24: 188-194. Ref.: https://goo.gl/rTFrX9
- Swislocki AM, Hoffman B, Reaven GM. Insulin resistance, glucose intolerance and hyperinsulinemia in patients with hypertension. Am J Hypertens. 1989; 2: 419-423. Ref.: https://goo.gl/Vz7a6B
- Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat Rev Cardiol. 2010; 7: 577-584. Ref.: https://goo.gl/cZbVcW
- Zhou MS, Schulman IH, Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc Med. 2012; 17: 330-341. Ref.: https://goo.gl/dQ2JuJ
- Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest. 1994; 94: 2511-2515. Ref.: https://goo.gl/smzbKp
- Manhiani MM, Cormican MT, Brands MW. Chronic sodium-retaining action of insulin in diabetic dogs. Am J Physiol Renal Physiol. 2011; 300: 957-965. Ref.: https://goo.gl/5ixsDL
- Chagas CA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type2 diabetes. Nutrients. 2012; 4: 52-67. Ref.: https://goo.gl/h9udS3
- Pavlov TS, Ilatovskaya DV, Levchenko V, Li L, Ecelbarger CM, et al. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J. 2013; 27: 2723-2732. Ref.: https://goo.gl/djgKrx
- Sone H, Ito M, Sugiyama K, Ohneda M, Maebashi M, et al. Biotin enhances glucose stimulated insulin secretion in the isolated perfused pancreas of the rat. J Nutri Biochem. 1999; 10: 237-243. Ref.: https://goo.gl/WYGeGZ
- Larrieta E, de la Vegal-Monroy ML, Vital P, Agiulera A, German MS, et al. Effects of biotin deficiency on pancreatic islet morphology, insulin sensitivity and glucose homeostasis. J Nut Biochem. 2012; 23: 392-399. Ref.: https://goo.gl/tAmXau
- Myers SA, Nield A, Myers M. Zinc transporters, mechanisms of action and therapeutic utility: implication for type2 diabetes mellitus. J Nutr Metab. 2012; Article ID 173712: 13. Ref.: https://goo.gl/SdvwgF
- Suâre A, Pulido N, Casla A, Casanova B, Ameta FJ, et al. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabeteologia. 1995; 38: 1262-1270. Ref.: https://goo.gl/MhMWpz
- Wang X, Zhang M, Lui G, Chang H, Zhang M, et al. Associations of serum manganese levels with prediabetes and diabetes among ≥ 60-year-old Chinese adults: A population–based cross-sectional analysis. nutrients. 2016; 8: 497. Ref.: https://goo.gl/WVmbE2
- Beck-Nielsen H. Insulin resistance: organ manifestation and cellular mechanism. Ugeskr Laeger. 2002; 164: 2130-2135. Ref.: https://goo.gl/Hspp2V
- Reaven GM. Insulin resistance and its consequences. In: Le Roith D, Taylor SI, Olefasky JM, eds. Diabetes mellitus: a fundamental and clinical text. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins. 2003; 899-915. Ref.: https://goo.gl/MJ6W85
- Godland IF, Crook D, Walton C, Wyon V, Oliver MF. Influence of insulin resistance, secretion and clearance on serum cholesterol, triglycerides, lipoprotein cholesterol and blood pressure in healthy men. Artherioscler Thromb. 1992; 12: 1030-1035. Ref.: https://goo.gl/n5DJSH
- Rodriquez E, Bermejo LM, Lopez-sobalar AM, Ortega RM. An inadequate intake of manganese may favour insulin resistance in girls. Nutr Hosp. 2011; 26: 965-970. Ref.: https://goo.gl/9cpbmU
- Rosario JF, Gomez MP, Anbu P. Does the maternal micronutrient deficiency (copper or zinc or vitamin E) modulate the expression of placental 11β hydroxysteriod dehydrogenase -2 per se predispose offspring to insulin resistance and hypertension in later life? Indian J Physiol Pharmacol. 2008; 52: 355-365. Ref.: https://goo.gl/pWnTpx
- Lee SH, Jouihan HA, Cooksey RC, Jones D, Kim HJ, et al. Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology. 2013; 154: 1029-1038. Ref.: https://goo.gl/RdN3D3
- Weidmann P, Bohlen L, de Courten M. Insulin resistance and hyperinsulinemia in hypertension. J Hypertens. 1996; 13: 65-72. Ref.: https://goo.gl/NxetKQ
- Salvetti A, Brogi G, di Legge V, Bernini GP. The interrelationship between insulin resistance and hypertension. Drugs. 1993; 46: 149-159. Ref.: https://goo.gl/uUkQgR
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 14: 840-846. Ref.: https://goo.gl/ViU9g5
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000; 106: 473-481. Ref.: https://goo.gl/ySbs9J
- Rao G. Insulin resistance syndrome. American Family Physician. 2001; 63: 1159-1163. Ref.: https://goo.gl/wLztk6
- Barker DJ. In utero programming of chronic disease. Clinical Science. 1998a; 95: 115-128. Ref.: https://goo.gl/c5UyYi
- Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005; 26: 19-39. Ref.: https://goo.gl/RvMC5h
- Quatanani M, Lazar MA. Mechanisms of obesity associated insulin resistance: many choices on the menu. GENES & Development. 2007; 21: 1443-1445. Ref.: https://goo.gl/Vo6AXm
- Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol 2012; Ref.: https://goo.gl/DXzLYo
- Rotruck JT, Hoekstra WG, Pope AL, Ganther H, Swanson A, et al. Relationship of selenium to GSH peroxidase Fed. Proc. 1973; 31; 691.
- Awasthi YC, Beutler E, Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975; 250: 5144-5149. Ref.: https://goo.gl/TJE1WR
- Schrauzer GN. Nutritional selenium supplementation; product types, quality, and safety. J Am Coll Nutr. 2001: 20; 1-4. Ref.: https://goo.gl/LhuFLB
- Thompson JN, Scott ML. Impaired lipid and vitamin E absorption related to atrophy of the pancrease in selenium-deficient chicks. J Nutr. 1970; 100: 797-809. Ref.: https://goo.gl/kHbghd
- Souness JE, Stouffer JE, Chagoya de Sanchez V. The effect of selenium-deficiency on rat fat-cell glucose oxidation. Biochem J. 1983; 214: 471-477. Ref.: https://goo.gl/mHBoCV
- Asayama K, Kooy NW, Burr IM. Effect of vitamin E deficiency and selenium deficiency on insulin secretory reserve and free radical scavenging systems in islets: decrease of islet manganosuperoxide dismutase. J Lab Clin Med. 1986; 107: 459–464. Ref.: https://goo.gl/Q5JvfS
- Navarro-Alarcon M, López-D de la Serrana H, Perez-Valero V, Lopez-Martinez C. Serum and urine selenium concentrations as indicators of body status in patients with diabetes mellitus. Sci Total Environ. 1999; 228: 79-85. Ref.: https://goo.gl/eZUikN
- Zhang MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012; 52: 1335-1342. Ref.: https://goo.gl/qmw59N
- Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, et al. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011; 14: 2327–2336. Ref.: https://goo.gl/XopaX7
- Diplock AT, Hoekstra WG. Metabolic Aspects of selenium action and toxicity critical review in toxicology. 1976; 4: 271-329. Ref.: https://goo.gl/pDQASx
- Zeng MS, Li X, Liu Y, Zhao H, Zhou JG, et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012; 52: 1335-1342. Ref.: https://goo.gl/Mu3gFP
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963; 1: 785-789. Ref.: https://goo.gl/6rEjvV
- Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006; 119: 10-16. Ref.: https://goo.gl/Hc3Lrw
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997; 46: 3-10. Ref.: https://goo.gl/4GfK8g
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006; 116: 1494-1505. Ref.: https://goo.gl/j5TW1A
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006; 116: 115-124. Ref.: https://goo.gl/4MjLVo
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117: 175-184. Ref.: https://goo.gl/ErtVpD
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000; 275: 9047-9054. Ref.: https://goo.gl/GF5oSY
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002; 420: 333-336. Ref.: https://goo.gl/ExxCsV
- Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004; 18: 2024-2034. Ref.: https://goo.gl/s4ykx2
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikk. Science. 2001; 293: 1673-1677 Ref.: https://goo.gl/5Vjgoy
- Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002; 277: 42394-42398. Ref.: https://goo.gl/BT8FBd
- Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci. 2004; 101: 10422-10427. Ref.: https://goo.gl/p2NX3J
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid- induced insulin resistance. J Clin Invest. 2006; 116: 3015-3025 Ref.: https://goo.gl/tUHe2H
- Zile MH. Vitamin A and embryonic development: An overview. J Nutr. 1988; 128: 455S-458S. Ref.: https://goo.gl/oQgDQj
- Martín M1, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, et al. Dorsal pancreas agenesis in retinoic acid-deficient RALDH2 mutant mice. Demonstrates the vital role of vitamin A in pancreatic development. Dev. Biol. 2005; 284: 399–411. Ref.: https://goo.gl/thgpTe
- Oström M1, Loffler KA, Edfalk S, Selander L, Dahl U, et al. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS ONE. 2008; 3: e2841. Ref.: https://goo.gl/jD8npN
- Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol 2009; 29: 3286–3296. Ref.: https://goo.gl/dmoeae
- Trasino SE, Gudas LJ. Vitamin A missing link in diabetes? Diabetes manag. 2015: 5: 359-367. Ref.: https://goo.gl/CC5BZj
- Amengual J, Ribot J, Bonet ML, Palou A. Retinoic acid treatment enhances lipid oxidation and inhibits lipid biosynthesis capacities in the liver of mice. Cell. Physiol. Biochem. 2010; 25: 657–666. Ref.: https://goo.gl/zdGWWq
- Transino SE, Benoit YD, Gudas LJ. Vitamin A deficiency causes hyperglycemia and loss of pancreatic β-cell mass. The Journal of Biological chemistry. 2015; 290: 1456-1473. Ref.: https://goo.gl/pxqX4i
- Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with Type 2 diabetes. Diabetologia. 2011; 54: 1720–1725. Ref.: https://goo.gl/JQJsja
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with Type 2 diabetes. Diabetes. 2003; 52: 102–110. Ref.: https://goo.gl/vLuDp5
- Yönen K, Alfthan G, Groop L, Saloranta C, Aro A, et al. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of Type 2 diabetes: the botnia dietary study. Am J Clin Nutr. 2003; 77: 1434–1441. Ref.: https://goo.gl/iQvMyK
- Sluijs I, Cadier E, Beulens JW, Van Der A DL, Spijkerman AM, et al. Dietary intake of carotenoids and risk of Type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015; 25: 376–381. Ref.: https://goo.gl/x2Aqif
- Basu TK, Tze WJ, Leichter J. Serum vitamin A and retinol-binding protein in patients with insulin-dependent diabetes mellitus. Am J Clin Nutr. 1989; 50: 329–331. Ref.: https://goo.gl/EGMWPe
- Lambrot R1, Xu C, Saint-Phar S, Chountalos G, Cohen T, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat commun4 2013. Ref.: https://goo.gl/su9Hbm
- Rosolová H, Simon J, Mayer O Jr, Racek J, Dierzé T, et al. Unexpected inverse relationship between insulin resistance and serum homocysteine in healthy subjects. Physiol Res. 2002; 51: 93-98. Ref.: https://goo.gl/NSjDqk
- Setola E, Monti LD, Galluccio E, Palloshi A, Fragassio G, Paroni R, et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patience with metabolic syndrome; relationship between homocysteine levels and hyper insulinemia. European Journal of Endocrinology. 2004; 151: 483-489. Ref.: https://goo.gl/5HNZnc
- Li J, Goh CE2, Demmer RT2, Whitcomb BW3, Du P, et al. Association between serum folate and insulin resistance among US Non-diabetic adults. Sci Rep. 2017; 7: 9187 Ref.: https://goo.gl/f5M1n4
- Najib S, Sánchez-Margalet V. Homocysteine thiolactone inhibits insulin signaling and glutathione has a protective effect. Journal of Molecular Endocrinology. 2001; 27: 85-91. Ref.: https://goo.gl/Di58WK
- Larrieta E, de la Vegal-Monroy ML, Vital P, Agiulera A, German MS, et al. Effects of biotin deficiency on pancreatic islet morphology, insulin sensitivity and glucose homeostasis. J Nut Biochem. 2012; 23: 392-399. Ref.: https://goo.gl/qLbL4U
- Myers SA, Nield A, Myers M. Zinc transporters, mechanisms of action and therapeutic utility: implication for type2 diabetes mellitus. J Nutr Metab. 2012; Article ID 173712: 13. Ref.: https://goo.gl/8EhfnY
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, et al. Endoplasmic reticulum stress link obesity, insulin action and type 2 diabetes. Science. 2004; 306: 457-461. Ref.: https://goo.gl/xHveD1
- Christie-David DJ, Girgis CM, Gunton JE. Effects of vitamin C and D in type 2 diabetes mellitus. Dovepress. 2015: 7; 21-28. Ref.: https://goo.gl/PGEJ1P
- Khodaeian M, Tabatabaer-Malazy O, Qorbani M, Farzadfar F, Amini P, et al. Effect of vitamin C and E on insulin resistance in diabetes: a meta-analysis study. Eur J. Clin Invest. 2015; 45: 1161-1174. Ref.: https://goo.gl/uzMJLi
- Picklo MJ, Thyfault JP. Vitamin E and vitamin C do not reduce insulin sensitivity but inhibit mitochondrial protein expression in exercising obese rats. Appl Physiol Nutr Metab. 2015; 40: 343-352. Ref.: https://goo.gl/9ix1Ve
- Johnston CS, Yen MF. Megadoses of vitamin C delays insulin response to a glucose challenge in normoglycemia adults. AM J. Clin. Nutr b. 1994; 60: 735-738. Ref.: https://goo.gl/C8upYc
- Ashor AW, Werner AD, Lara J, Willis ND Mathers JC, Siervo M. Effects of vitamin C supplementation on glycaemic control: a Systematic review and meta-analysis of renal missed controlled trials. Eur J Clin Nutr. 2017; 71: 1371-1380. Ref.: https://goo.gl/8Sd5AJ
- Mason SA, Della Gatta PA, Snow RJ, Russell AP, Wadley GD. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes. Findings of a randomized controlled study. Free Radical Biol. Med. 2016; 93: 227-238. Ref.: https://goo.gl/fMHZrF
- Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009; 15: 1097-1110. Ref.: https://goo.gl/ZABrRD
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors cause hyperphagia and insulin resistance in rats. Nat Neurosci. 2002a; 5: 566-572. Ref.: https://goo.gl/VPkZrg
- Obici S, Feng Z, Morgan K, Stein D, Karkanias G, et al. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002b; 51: 271-275 Ref.: https://goo.gl/mymijQ
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, et al. Free fatty acid induced insulin resistance is associated with activation of phosphatidylinositol 3-kinase. Diabetes. 1999; 48: 1270-1274. Ref.: https://goo.gl/sqywhK
- Kim J, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008; 102: 401-414. Ref.: https://goo.gl/GsqKiv
- Kyriakis JM, Avruch J. Sounding the alarm: Protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996; 271: 24313-24316. Ref.: https://goo.gl/UxCoF6
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress activated signaling pathways mediators of insulin resistance and beta dysfunction? Diabetes. 2003; 52: 1-8. Ref.: https://goo.gl/Rq4vMf
- Morino K, Peterson KF, Dufour S, Befroy D, Frattini J, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin resistant offspring of type2 diabetes parents. J Clin Invest. 2005; 115: 3587-3593. Ref.: https://goo.gl/pgpuQV
- Eckel RH, Prasad PA, Kern PA, Marshall S. Insulin regulation of lipoprotein lipase in cultured isolated rats adipocytes. Endocrinology. 1984; 114: 1665-1671. Ref.: https://goo.gl/L2wHSD
- Shidfar F, Rezael KH, Hosseini S, Heydari. The effect of vitamin E on insulin resistance and cardiovascular diseases risk factors in metabolic syndrome. Iranian J of Endocrinology and metabolism (IJEM). 2009; 10: 445-454. Ref.: https://goo.gl/mNqBjC
- Moorthi RV, Bobby Z, Selvaraj N, Sridhar MG. Vitamin E protects the insulin sensitivity and redox balance in rat L6 muscle cells exposed to oxidative stress. Clinica Chemica Acta. 2006; 367:132-136. Ref.: https://goo.gl/VtP3pv
- Manning PJ, Sutherland WH, Williams SM, DeJong SA, Ryalls AR, et al. Effect of high dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes care. 2004: 27: 2166-2171. Ref.: https://goo.gl/QW1ZGL
- Sanchez-Lugo L, Mayer-George EJ, Howard G, Selby JV, et al. Insulin sensitivity and intake of vitamins E and C in African. American Hispanic and non-Hispanic white men and women. The insulin resistance and atherosclerosis study (IRAS). Am J Clin Nutr. 1997; 66: 1224-1231. Ref.: https://goo.gl/sWupAx